CNS Targeting Antibody-coupled Liposome Development Service
In the ever-evolving landscape of drug delivery and targeted therapies, the quest for precision and efficacy is paramount, particularly in the field of nephrology. As a leading Contract Research Organization (CRO) specializing in drug formulation and delivery systems, Creative Biolabs is happy to introduce our innovative approach to treating central nervous system (CNS) diseases-CNS Targeting Antibody-coupled Liposomes. Our CNS-targeting antibody-coupled liposomes represent a novel drug delivery system specifically designed for the targeted delivery of therapeutic agents to CNS.
Introduction
Globally, the incidence of CNS diseases is on the rise. However, the development of CNS drugs remains particularly challenging, characterized by high costs, lengthy research timelines, and elevated failure rates. Creative Biolabs has consistently focused on studying how CNS physiology impacts the delivery of drugs to the brain and spinal cord. Consequently, we have designed and developed a variety of strategies and materials for CNS drug delivery. Among them, our biomaterials can bypass or penetrate these barriers, enabling controlled delivery of drugs to the CNS.
Transferrin Receptor 1 (TfR1) is a particularly appealing target within the RMT system and is one of the most extensively studied systems for drug delivery to CNS. Creative Biolabs utilizes this system for targeted CNS drug transport by either targeting transferrin (Tf) or designing antibodies specifically against TfR1. Moreover, our CNS-targeting antibody-coupled liposome development services involve a full range of systems for delivering therapeutic agents directly to the brain or spinal cord.
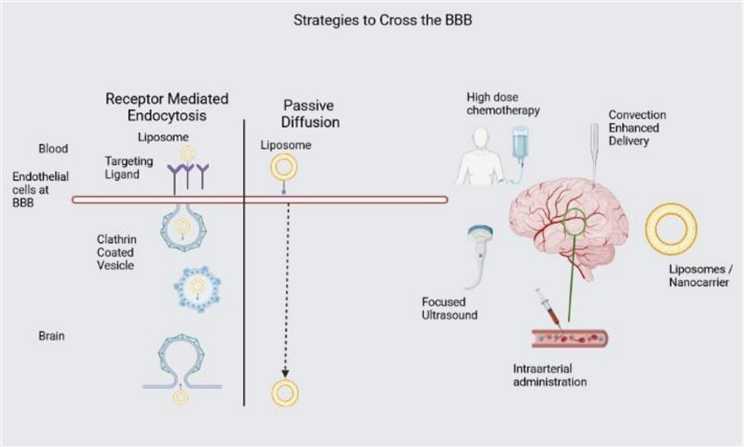 Fig.1 Strategies to Cross the BBB.1,3
Fig.1 Strategies to Cross the BBB.1,3
Services
- TfR1 Antibody liposomal Brain Delivery Services
Recently, Creative Biolabs has specialized in drug delivery systems and can assist with the formulation, testing, and optimization of liposomal carriers aimed at targeting TfR1. We have incorporated TfR1 antibodies onto the surface of liposomes to create a molecular Trojan horse structure that binds to TfR1, triggering the transport of nano drug particles across the blood-brain barrier (BBB). This system enables targeted delivery of liposome-encapsulated drugs to the brain. For example, we used this method to deliver plasmid DNA encapsulated in liposomes to the brains of mice to express it for the treatment of NPC1, a hereditary lysosomal storage disease. Simultaneously, we enhanced drug delivery to the brain using docetaxel (DTX) liposomes co-modified with muscone and the TfR antibody (RI7217) to achieve anti-glioma effects. OX26-modified nanostructured lipid carriers (NLC) can facilitate the brain delivery of salvianolic acid B (Sal B) and baicalin (BA), aiding in the repair of neuronal damage and improving outcomes following cerebral ischemia-reperfusion injury (IRI). Furthermore, by adjusting the density of TfR antibodies on the surface of nanoparticles, we can finely tune their absorption and transport processes to the brain, while also reducing the risk of serious adverse reactions that TfR-targeted liposomes may cause after intravenous administration in mice.
- CNS Targeting Antibody-Coupled Liposome Services
In our labs, CNS-targeting antibody-coupled liposomes have developed a powerful tool for drug delivery, aiming to improve the treatment of CNS disorders. Certain types of liposomes are designed to transport therapeutic agents, such as drugs or genes, and are coated with antibodies that can selectively bind to receptors or antigens present on the surface of CNS cells. Here are some essential aspects of developing CNS-targeting antibody-coupled liposome services:
1. Liposome Design
Composition: Selecting appropriate lipids to formulate stable liposomes that efficiently encapsulate therapeutic agents, including small molecules, peptides, and nucleic acids.
Formulation Development: Fine-tuning the size, charge, and stability of liposomes to enhance delivery while minimizing immune responses.
2. Antibody Coupling
Antibody Selection: Identifying or creating antibodies that specifically target receptors or proteins located on the surface of CNS cells, including neurons and glial cells.
Conjugation Techniques: Utilizing various methods for attaching antibodies to the liposome surface, employing both covalent and non-covalent strategies to maintain antibody functionality.
3. Targeting Strategy
Receptor-Ligand Interactions: Exploiting specific interactions between receptors and ligands to facilitate endocytosis or uptake by targeted CNS cells.
Screening and Validation: Evaluating the binding efficiency and biological activity of antibody-coupled liposomes through in vitroexperiments and animal model studies.
4. In Vitro and In Vivo Testing
Cell Culture Studies: Testing the efficacy of liposome formulations in cell lines that accurately represent CNS biology.
Animal Models: Conducting pharmacokinetics and biodistribution studies, as well as therapeutic efficacy assessments, using appropriate disease models in animals.
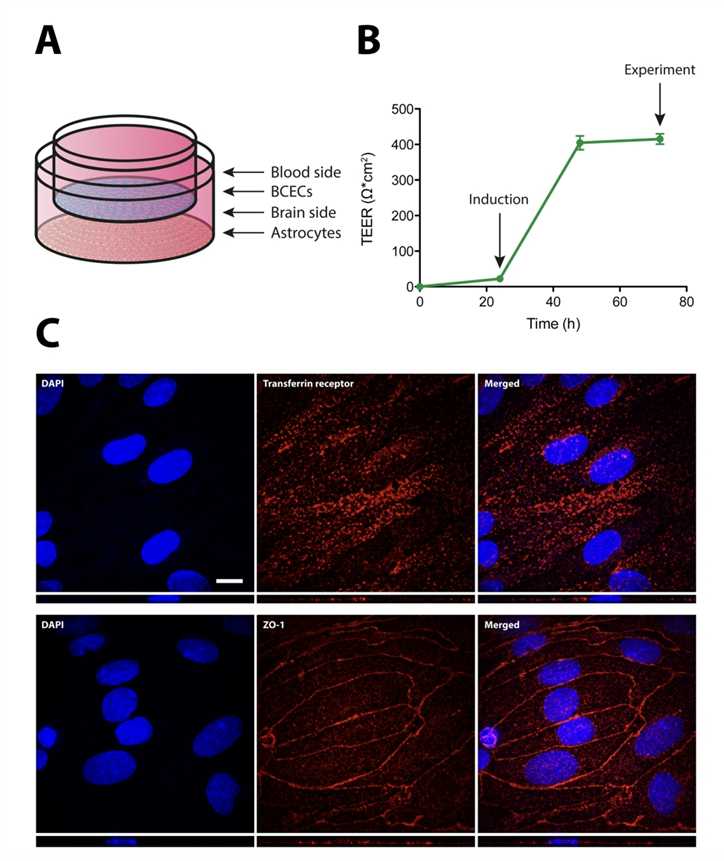 Fig.2 Characteristics of the Immunoliposomes BBB Crossing.2,3
Fig.2 Characteristics of the Immunoliposomes BBB Crossing.2,3
Advantages
- Enhanced BBB Penetration: Antibody-conjugated liposomes can be designed to target specific receptors or antigens present on the endothelial cells that comprise the BBB, thus facilitating the delivery of therapeutic agents across this otherwise protective barrier.
- Improved Targeting Specificity: Attaching antibodies that selectively bind to central nervous system (CNS) targets, such as tumor antigens or neuronal receptors, enhances targeting precision, which minimizes off-target effects and raises the concentration of the drug in the affected area.
- Reduced Systemic Toxicity: Targeted delivery to the CNS decreases systemic exposure to therapeutic agents, resulting in fewer side effects and reduced toxicity compared to traditional systemic administration.
- Increased Drug Efficacy: Administering drugs directly to the site of action within the CNS can boost their therapeutic effectiveness by achieving higher local concentrations while minimizing required dosages.
- Versatile Drug Delivery Platform: Liposomes can encapsulate a wide range of drugs, including small molecules, peptides, proteins, and nucleic acids, making them a flexible platform for drug development.
- Controlled Release Properties: Liposomes can be engineered for controlled release of their payload, extending the therapeutic effect and lessening the need for frequent dosing.
- Potential to Overcome Resistance Mechanisms: Targeted liposomes may circumvent specific resistance mechanisms faced by conventional therapies, especially in the treatment of CNS tumors and neurodegenerative diseases.
Creative Biolabs can design and develop advanced liposomal formulations that are conjugated with targeted antibodies. Our advanced approach enhances the bioavailability of pharmaceuticals in the CNS, optimizing therapeutic efficacy while reducing systemic side effects. We prioritize a collaborative partnership with our clients, ensuring clear communication and customized support throughout the whole process to effectively and efficiently achieve project goals. We invite you to contact us for more information.
- Raju, Richu, et al. "Liposomes for the treatment of brain cancer-A review." Pharmaceuticals 16.8 (2023): 1056.
- Johnsen, Kasper Bendix, et al. "Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma." Scientific Reports 7.1 (2017): 1-13.
- Distributed under Open Access license CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

