Fab Antibody Fragment Production and QC Service
Fab antibody fragments (Fragments of Antigen Binding) are gaining increasing attention due to their high specificity and excellent affinity. If you require efficient and reliable services for the expression and purification of Fab antibody fragments, Creative Biolabs warmly invites you to collaborate with us to enhance the smoothness of your research and development journey.
Introduction
In recent years, there has been growing interest in Fab antibody fragments, which are recognized for their excellent biocompatibility and biological activity, particularly as monoclonal antibodies and their derivatives have become widely used in therapies. Fab fragments can selectively bind to antigens while minimizing the risk of precipitation reactions. Furthermore, their relatively low immunogenicity means that they are less likely to be recognized by in vivo immune cells, significantly reducing the risk of hypersensitivity reactions and enhancing product safety. These Fab antibody fragments not only have a smaller molecular weight and specific tissue distribution but also exhibit low immunogenicity and the potential for genetic engineering modifications. Therefore, this combination of characteristics positions Fab fragments as a key element in pharmaceutical research.
As a leading CRO company, Creative Biolabs employs genetic engineering techniques to express Fab antibody fragments in host cells such as Escherichia coli, yeast, or mammalian cells. We assist clients in designing specific DNA sequences that encode the Fab fragments, which are tailored to recognize specific antigens. Once these genes are introduced into the host cells through transfection or transformation methods, we create optimal culture conditions to promote the expression of the target Fab. After expression, we purify the Fab fragments using methods such as affinity chromatography, ion exchange chromatography, or gel filtration, ensuring the production of high-purity and high-quality antibody samples.
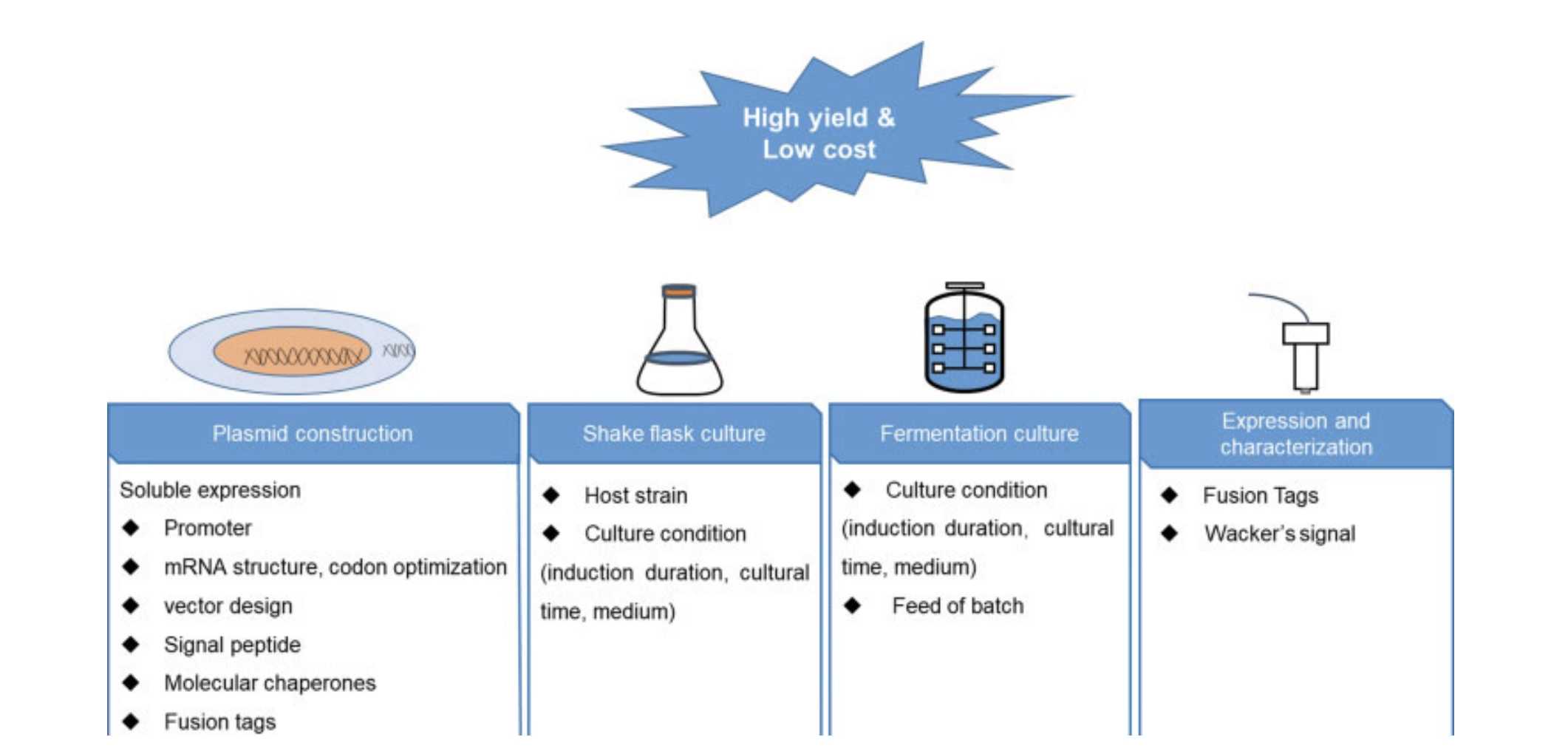 Fig.1 E. coli System for Fab Fragments Production.1,3
Fig.1 E. coli System for Fab Fragments Production.1,3
Services
Currently, Creative Biolabs offers a wide range of expression and purification solutions for Fab antibody fragments to support the needs of our valued clients. In our labs, we have generated various methods for preparing and purifying Fab fragments, including but not limited to:
- Enzymatic Digestion: Typically, we use papain or pepsin to degrade immunoglobulin G, which allows us to obtain Fab/F(ab')2 and Fc fragments.
- Expression Systems for Fab Fragment Production: Our laboratory currently employs both Escherichia coli and mammalian cell expression systems. The E. coli expression system is cost-effective and enables rapid production; however, it often results in the formation of inclusion bodies, making it challenging to ensure the bioactivity after refolding. In contrast, mammalian cell expression systems can effectively form disulfide bonds, allowing the generated Fab fragments to closely resemble their natural structure, leading to superior activity.
- Phage Display Platform: We can construct a Fab fragment antibody library using this approach, followed by multiple rounds of selection to enrich for high-affinity antibodies. This method provides an effective means for screening antibodies with high specificity.
- Affinity Chromatography: We use the unique binding properties of antibody fragments to achieve initial purification by interacting with a resin that contains the target ligand.
- Ion Exchange Chromatography: Our technique facilitates further purification based on the charge characteristics of the Fab fragments, effectively removing non-specifically bound impurities.
- Gel Filtration Chromatography: Utilizing differences in molecular weight, our scientists can enhance the purity of Fab fragments further.
- Protein Modifications and Folding: In certain instances, our expressed Fab fragments may require post-translational modifications, such as glycosylation or disulfide bond formation, to enhance their stability and biological activity. We can further ensure the proper folding of Fab fragments by offering optimized culture conditions and using cofactors among our services.
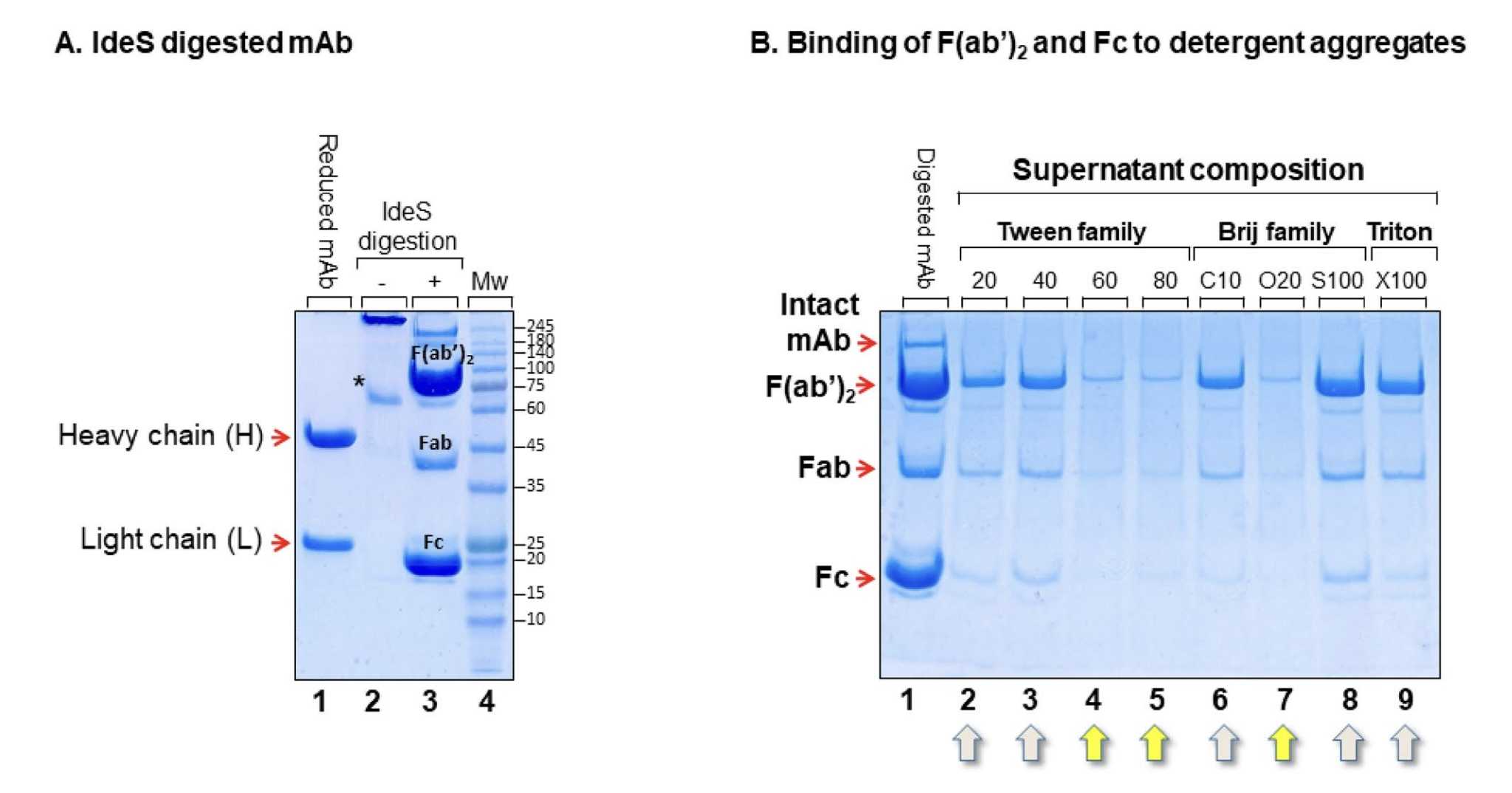 Fig.2 A Fab Fragments Purification Strategy.2,3
Fig.2 A Fab Fragments Purification Strategy.2,3
Advantages
- By optimizing the design, we ensure the acquisition of a high-expression and highly specific Fab antibody framework, which we then clone into suitable expression vectors.
- Our technical team systematically optimizes the expression conditions for the Fab antibody fragments, including variables like temperature, culture media, and induction time, to guarantee the highest possible protein yield.
- All produced Fab antibody fragments undergo stringent quality control measures, utilizing techniques such as SDS-PAGE, Western Blot, and ELISA to verify their purity, specificity, and functional activity.
- Creative Biolabs offers a versatile service model. Whether for small-scale pilot production or large-scale manufacturing, we can adapt our services to meet the specific needs of our clients. Additionally, we provide tailored solutions to address particular research and development requirements under unique conditions.
- We have streamlined our workflows to minimize delivery times as much as possible. With effective project management and technical support, we can efficiently complete the expression and purification of antibody fragments in a shorter timeframe, ensuring that project demands are met.
Creative Biolabs provides rapid and accurate solutions for the expression and purification of Fab antibody fragments. We can deliver highly pure and high-yield antibody fragments in a short timeframe. Whether you require a bacterial expression system, a eukaryotic expression system, or advanced chromatography purification techniques, we are here to provide the optimal solution for you. We invite you to contact us to discuss your Fab antibody fragment expression and purification needs and project objectives.
- Chen, Hui, et al. "Strategies and applications of antigen-binding fragment (Fab) production in Escherichia coli." Pharmaceutical Fronts 3.02 (2021): e39-e49.
- Dhandapani, Gunasekaran, et al. "Purification of antibody fragments via interaction with detergent micellar aggregates." Scientific Reports 11.1 (2021): 11697.
- Distributed under Open Access license CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

