IgA1 O-Glycan Modification Service
Background
IgA1's hinge region is uniquely characterized by multiple O-glycosylation sites, where complex saccharide chains are attached to serine and threonine residues. These O-Glycans, typically composed of N-acetylgalactosamine (GalNAc), galactose, and sialic acid, exhibit remarkable micro- and macro-heterogeneity. This structural diversity, arising from the stepwise enzymatic addition of sugars, is not incidental; it profoundly influences IgA1's structural integrity, proteolytic susceptibility, and critical effector functions, including interactions with immune cells and complement activation.
The profound significance of IgA1 O-glycosylation becomes strikingly evident in the context of disease. IgA nephropathy (IgAN), the most prevalent primary glomerular disease worldwide, serves as a quintessential example. In IgAN, a hallmark molecular abnormality is the presence of galactose-deficient IgA1 (Gd-IgA1). This aberrant glycoform, characterized by a reduction or absence of galactose residues in the hinge region O-Glycans, is recognized by circulating autoantibodies. The subsequent formation of pathogenic immune complexes, which deposit in the kidney's mesangium, triggers an inflammatory cascade leading to progressive renal damage. Elevated serum levels of Gd-IgA1 are a consistent finding in IgAN patients, correlating directly with disease severity and progression, thereby establishing Gd-IgA1 as a crucial biomarker for diagnosis and prognosis. Beyond IgAN, altered IgA1 O-glycosylation patterns are increasingly implicated in other immune-mediated and inflammatory conditions, underscoring the broader relevance of this molecular signature in disease pathology.
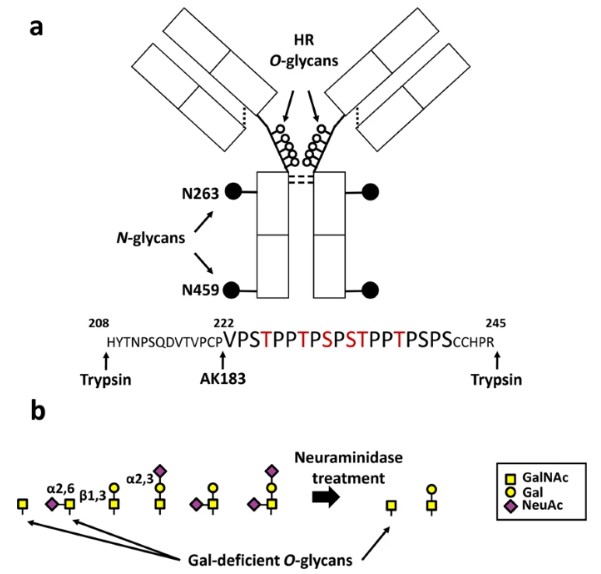 Fig.1 IgA1 structure and the hinge-region (HR) O-Glycans.1
Fig.1 IgA1 structure and the hinge-region (HR) O-Glycans.1
Comprehensive IgA1 O-Glycan Modification Service at Creative Biolabs
Recognizing the critical insights offered by detailed IgA1 O-Glycan analysis, Creative Biolabs has developed a state-of-the-art service specifically designed to comprehensively characterize these complex modifications. Our service is indispensable for researchers and clinicians aiming to:
- Elucidate Disease Mechanisms: Pinpoint the precise molecular alterations in IgA1 glycosylation that drive pathological processes.
- Discover Novel Biomarkers: Identify specific IgA1 glycoforms or glycosylation patterns with diagnostic, prognostic, or predictive potential.
- Guide Therapeutic Development: Inform the design of targeted therapies by understanding the enzymatic and cellular pathways that regulate IgA1 glycosylation.
Our specialized expertise at Creative Biolabs ensures that clients receive unparalleled depth of insight into the intricate world of IgA1 O-Glycans, enabling breakthroughs in their respective fields.
Advanced Service Strategy for IgA1 O-Glycan Modification
Our IgA1 O-Glycan Modification service employs a meticulously optimized, multi-faceted strategy that integrates cutting-edge analytical technologies with advanced bioinformatics, providing a comprehensive and highly precise view of IgA1 glycosylation. Creative Biolabs leverages a suite of high-resolution mass spectrometry platforms, each chosen for its unique capabilities in deciphering the intricate details of IgA1 O-glycosylation:
Liquid Chromatography-High Resolution Mass Spectrometry (LC-HRMS)
This foundational technique provides unparalleled sensitivity and mass accuracy for the separation and identification of IgA1 O-glycopeptides. It enables site-specific resolution, allowing for the precise identification and quantification of individual glycoforms attached to specific amino acid residues within the hinge region.
Matrix-Assisted Laser Desorption/Ionization-Time-of-Flight/Time-of-Flight (MALDI-TOF/TOF) Mass Spectrometry
This platform is utilized for rapid, high-throughput profiling of IgA1 O-Glycan heterogeneity. It is particularly effective for characterizing the overall glycan repertoire and identifying isomeric O-glycoforms, providing a broad overview of the glycosylation landscape.
Electron-Transfer/Higher-Energy Collision Dissociation (EThcD) Tandem Mass Spectrometry
For unambiguous localization of glycan attachment sites and detailed structural elucidation, we employ advanced fragmentation techniques like EThcD. This method provides highly informative fragmentation patterns of glycopeptides, allowing for the precise assignment of glycans to specific serine or threonine residues within the IgA1 hinge region. This site-specific information is paramount for understanding the functional consequences of glycosylation changes.
Data-Independent Acquisition (DIA) Mass Spectrometry
To address the need for high-throughput and quantitative analysis in large-scale studies, Creative Biolabs integrates DIA strategies (e.g., SWATH-MS). Unlike traditional data-dependent acquisition, DIA acquires data on virtually all detectable ions in a sample, leading to superior reproducibility, more comprehensive coverage, and robust quantification across extensive sample cohorts. This capability is crucial for biomarker discovery and validation efforts.
Our Advantages
Creative Biolabs stands as a leader in the field of glycomics, offering unparalleled advantages to our clients:
- Unrivaled Expertise
- Cutting-Edge Technological Infrastructure
- Precision and High-Throughput Capability
- Deep Mechanistic Insights
FAQs
Q1: What is IgA1 O-glycosylation and why is it important?
A1: IgA1 O-glycosylation refers to the attachment of sugar chains (O-Glycans) to specific serine and threonine residues in the hinge region of the IgA1 antibody. These modifications are crucial for IgA1's structure, stability, and immune functions. Aberrant O-glycosylation, particularly galactose deficiency (Gd-IgA1), is a key pathogenic factor in diseases like IgA nephropathy (IgAN).
Q2: What kind of data and insights will I receive from Creative Biolabs' service?
A2: Clients receive detailed reports including the identification and relative quantification of specific IgA1 O-glycoforms, their precise attachment sites within the hinge region, and the overall heterogeneity of the IgA1 glycome. We can also provide correlations with clinical parameters and insights into potential mechanistic drivers of observed changes.
Q3: Can Creative Biolabs assist with other protein glycosylation analyses beyond IgA1?
A3: Yes, Creative Biolabs' expertise extends to a broad range of protein glycosylation analyses, including N-glycosylation and other O-glycosylation types, across various proteins and biological systems. Our advanced platforms and experienced team are equipped to tackle diverse glycomics challenges.
Contact Us
To learn more about how Creative Biolabs' unparalleled expertise in IgA1 O-Glycan Modification analysis can advance your research and development initiatives, please contact us today. Our scientific team is ready to discuss your specific project needs and design a customized solution that delivers precise, actionable insights.
Reference
- Ohyama, Yukako, et al. "Analysis of O-glycoforms of the IgA1 hinge region by sequential deglycosylation." Scientific reports 10.1 (2020): 671. Distributed under Open Access License CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

