IgA Bispecific Fcαri-Targeting Production Service
Introduction: Harnessing the Power of IgA for Targeted Immunotherapy
The landscape of therapeutic antibodies is rapidly evolving, with bispecific antibodies emerging as a frontier for highly targeted interventions. Among these, IgA bispecific antibodies targeting Fcαri (CD89) represent a particularly compelling class, designed to simultaneously engage two distinct targets: a specific disease associated antigen and the Fcαri receptor. This receptor is predominantly expressed on key myeloid immune effector cells, including neutrophils and macrophages, making it an ideal conduit for redirecting the innate immune system. At Creative Biolabs, with over two decades of specialized experience in advanced biologics, we recognize the profound potential of this dual-targeting mechanism to precisely guide immune effector cells to sites of pathology, thereby enhancing the elimination of diseased cells, particularly in oncology and infectious diseases.
The unique properties of IgA, distinct from IgG, are central to this therapeutic strategy. While IgG-mediated lysis often relies on complement-dependent mechanisms, IgA-dependent killing is primarily effector cell mediated, with neutrophils playing a crucial role via Fcαri. This fundamental difference underscores the importance of understanding and leveraging the specific immunological pathways activated by IgA. Our deep expertise at Creative Biolabs allows us to navigate these complexities, offering unparalleled production services for these sophisticated molecules.
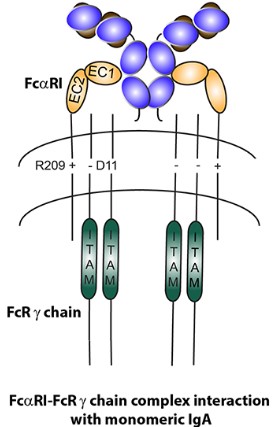 Fig.1 Structure of IgA Fc receptor (FcαRI).1
Fig.1 Structure of IgA Fc receptor (FcαRI).1
Our IgA Bispecific Fcαri-Targeting Production Service
The development of IgA bispecific Fcαri-targeting antibodies is a complex undertaking, demanding advanced genetic engineering, optimized expression systems, and stringent purification protocols. These molecules are inherently more intricate than traditional monoclonal antibodies, necessitating specialized expertise to ensure correct heavy and light chain pairing, structural integrity, and functional efficacy. Creative Biolabs stands at the forefront of this challenging field, providing a comprehensive production service designed to overcome these complexities and accelerate the translation of these innovative biologics from concept to clinic.
Our service is meticulously structured to address every critical phase of IgA bispecific antibody development, from initial design to final characterization. We leverage our extensive experience and cutting-edge technologies to deliver high-quality, functionally active IgA bispecific antibodies, enabling our partners to push the boundaries of immunotherapy.
Service Content and Strategy
Creative Biolabs' IgA Bispecific Fcαri-Targeting Production Service encompasses a full spectrum of capabilities, meticulously designed to ensure the successful development and manufacturing of these advanced therapeutic antibodies.
Precision Engineering of Antibody Fragments
The foundation of any successful bispecific antibody lies in its precise molecular engineering. Our service begins with the sophisticated design of variable regions to ensure high-affinity binding to both the desired disease-associated antigen and the Fcαri receptor. Concurrently, constant regions are meticulously modified to facilitate proper assembly and stability. We employ state-of-the-art genetic engineering techniques, including advanced strategies such as "knobs-into-holes," common light chain, technologies. These methods are critical for improving bispecific antibody assembly, minimizing mispairing, and enhancing overall product yield and homogeneity. Our proficiency in these techniques is a cornerstone of Creative Biolabs' commitment to delivering structurally sound and functionally superior molecules.
Optimized Expression in High-Yield Host Systems
Following engineering, the engineered genetic material is introduced into carefully selected host cells for robust protein production. Creative Biolabs utilizes optimized host cell platforms, primarily advanced mammalian cell lines such as Chinese Hamster Ovary (CHO) and Human Embryonic Kidney 293 (HEK293) cells, known for their capacity to produce complex glycoproteins with appropriate post-translational modifications. For specific applications, we also explore cell-free expression systems, offering flexibility and rapid production timelines. The selection and optimization of the expression system are paramount for enhancing antibody production yields, ensuring correct folding, and maintaining the desired quality attributes of the bispecific antibody.
Rigorous Purification and Comprehensive Characterization
The production of bispecific antibodies necessitates highly refined purification methods to isolate the target molecule from impurities and potential byproducts. Our purification protocols are tailored to the specific properties of each bispecific antibody, employing advanced chromatographic techniques to achieve exceptional purity. Post-purification, each batch undergoes comprehensive characterization to ensure quality and functionality. This includes detailed analyses of molecular weight, valency, and spatial arrangement of binding sites, which are critical influencers of stability and function. Furthermore, we meticulously assess for stability and aggregation propensity, employing various analytical techniques to guarantee product integrity throughout its lifecycle. Correct heavy and light chain pairing is rigorously verified, as mispairing can significantly compromise therapeutic efficacy.
Strategic Immunogenicity Reduction
The potential for therapeutic antibodies to elicit an immune response in patients is a critical consideration. Creative Biolabs integrates strategic immunogenicity reduction techniques into the design and production process. This includes humanization techniques and epitope modification, aimed at minimizing the immunogenic potential of the IgA bispecific antibody while preserving its therapeutic efficacy. Our proactive approach to immunogenicity ensures a safer and more effective therapeutic profile.
Target Antigen Selection and Dosing Protocol Support
Beyond antibody production, Creative Biolabs offers consultative support in critical upstream and downstream processes. We provide guidance in identifying and validating appropriate target antigens that offer genuine therapeutic benefit, a crucial prerequisite for successful drug development. Furthermore, our team assists in determining optimal dosing protocols, aiming to achieve the desired therapeutic effect while minimizing potential adverse events, drawing upon our extensive knowledge of pharmacokinetics and pharmacodynamics.
Our Advantages
At Creative Biolabs, our two decades of leadership in biological solutions translate into distinct advantages for our partners seeking to develop IgA bispecific Fcαri -targeting antibodies.
- Pioneering Expertise in Fcαri Biology
- Advanced Genetic Engineering and Assembly Technologies
- Optimized Expression and Scalability
- Rigorous Quality Control and Stability Assurance
- Insights into In Vivo Efficacy and Synergistic Strategies
FAQs
Q1: What are the potential applications of IgA bispecific Fcαri-Targeting antibodies?
A1: These antibodies hold significant therapeutic potential across various disease areas. Their primary application is in oncology, where they can redirect immune cells to kill tumor cells. They also show promise in infectious diseases by enhancing phagocytosis of pathogens and in inflammatory and autoimmune conditions where modulation of the IgA-Fcαri axis can mitigate tissue damage.
Q2: Why is Fcαri a promising therapeutic target?
A2: Fcαri is a compelling target because of its expression on key cytotoxic immune effector cells. Engaging Fcαri directly activates these cells, leading to potent antibody-dependent cell-mediated cytotoxicity (ADCC) and phagocytosis. This mechanism allows for the direct redirection of innate immune responses to specific pathological sites, offering a novel approach to immunotherapy.
Q3: What are the main challenges in producing these antibodies?
A3: The production of IgA bispecific Fcαri-targeting antibodies faces several challenges due to their structural complexity. These include ensuring correct heavy and light chain pairing, maintaining stability and preventing aggregation, and minimizing potential immunogenicity. Manufacturing complexity is also a significant factor compared to traditional monoclonal antibodies.
Q4: How does Creative Biolabs address these production challenges?
A4: Creative Biolabs addresses these challenges through a multi-faceted approach. We utilize advanced genetic engineering techniques (e.g., "knobs-into-holes," common light chain) to improve assembly and reduce mispairing. We employ optimized host cell platforms for enhanced production and quality. Our rigorous purification methods and comprehensive characterization ensure product integrity and functionality. Furthermore, we integrate immunogenicity reduction strategies into the design process.
Contact Us
To learn more about Creative Biolabs' IgA Bispecific Fcαri-Targeting Production Service and how we can support your next-generation therapeutic development, please contact our expert team. We are ready to collaborate and bring your innovative biologics to fruition.
Reference
- Breedveld, Annelot, and Marjolein van Egmond. "IgA and FcαRI: Pathological Roles and Therapeutic Opportunities." Frontiers in immunology vol. 10 553. 22 Mar. 2019. DOI: 10.3389/fimmu.2019.00553. Distributed under Open Access License CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

