Cell Depleting Antibody Production Service
Are you currently facing long drug development cycles, challenges in antibody development, or the need for highly specific and effective therapeutic agents? Our cell depleting antibody production service helps you accelerate therapeutic antibody development and obtain high-quality, efficacious, and safe cell-depleting antibodies through advanced Fc engineering strategies, comprehensive functional characterization, and robust production platforms.
Introduction
Cell-depleting antibodies represent a cornerstone of modern immunotherapy, precisely targeting and eliminating specific cell populations implicated in disease. This therapeutic strategy has revolutionized the treatment of autoimmune disorders and various cancers by leveraging the body's own immune mechanisms. Through sophisticated Fc-engineering, these antibodies are designed to maximize effector functions like antibody dependent cell-mediated cytotoxicity (ADCC), complement dependent cytotoxicity (CDC), antibody dependent cellular phagocytosis (ADCP), or direct apoptosis, offering highly effective and tailored solutions. Creative Biolabs' service is at the forefront of this innovation, ensuring the development of potent and safe therapeutic antibodies.
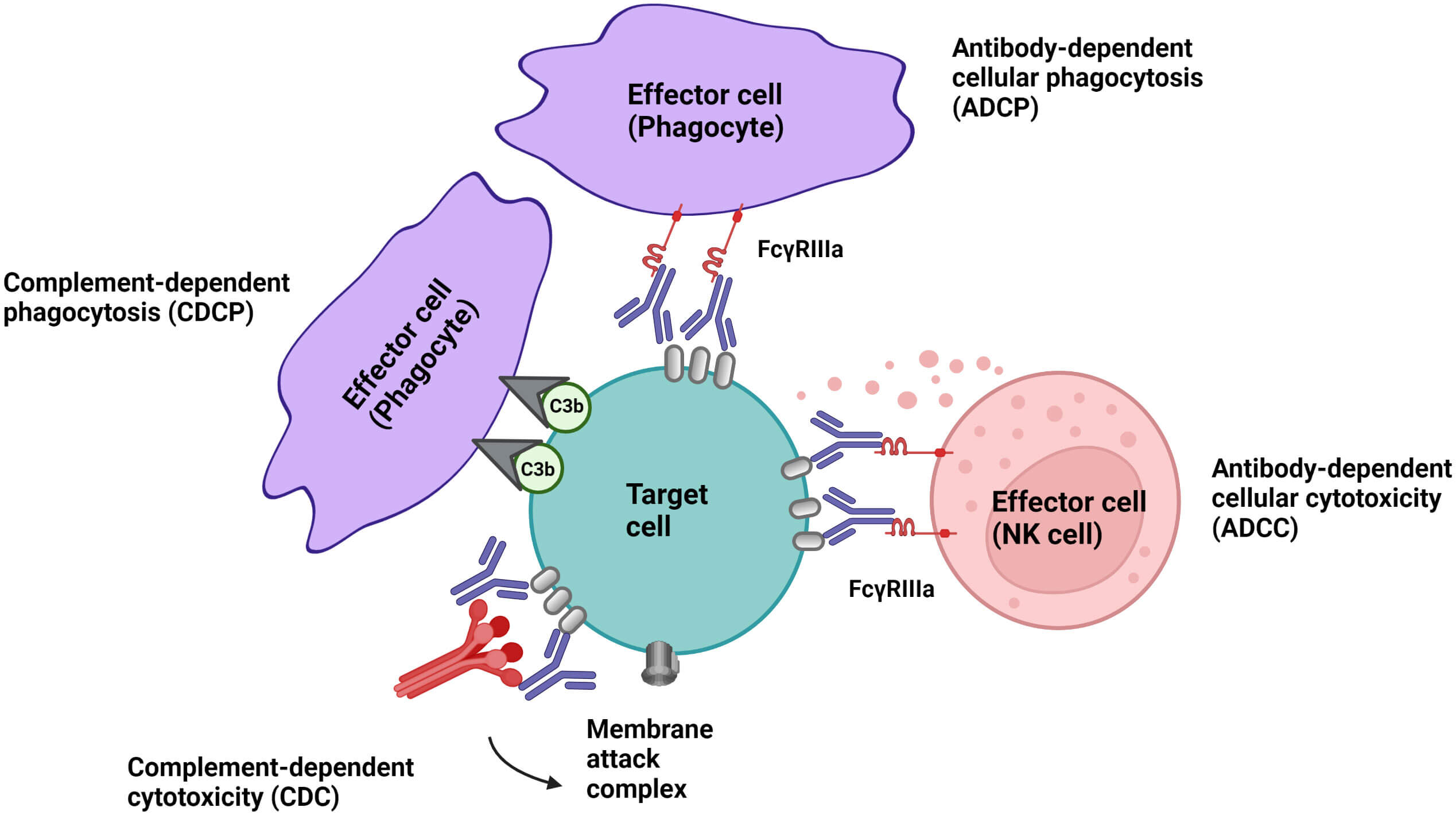 Fig.1 Multiple mechanisms of cell-depleting antibodies.1,3
Fig.1 Multiple mechanisms of cell-depleting antibodies.1,3
Our Services
At Creative Biolabs, we deliver bespoke solutions tailored to the unique demands of your therapeutic antibody programs. Our cell depleting antibody production service provides precisely engineered antibodies, optimized for potent effector functions and robust performance in preclinical and clinical settings. You can expect antibodies with enhanced ADCC, CDC, ADCP, or direct apoptotic capabilities, rigorously characterized for purity, specificity, and activity. Our solutions are designed to overcome the inherent limitations of native antibodies, accelerating your journey from concept to clinic.
Workflow Procedures
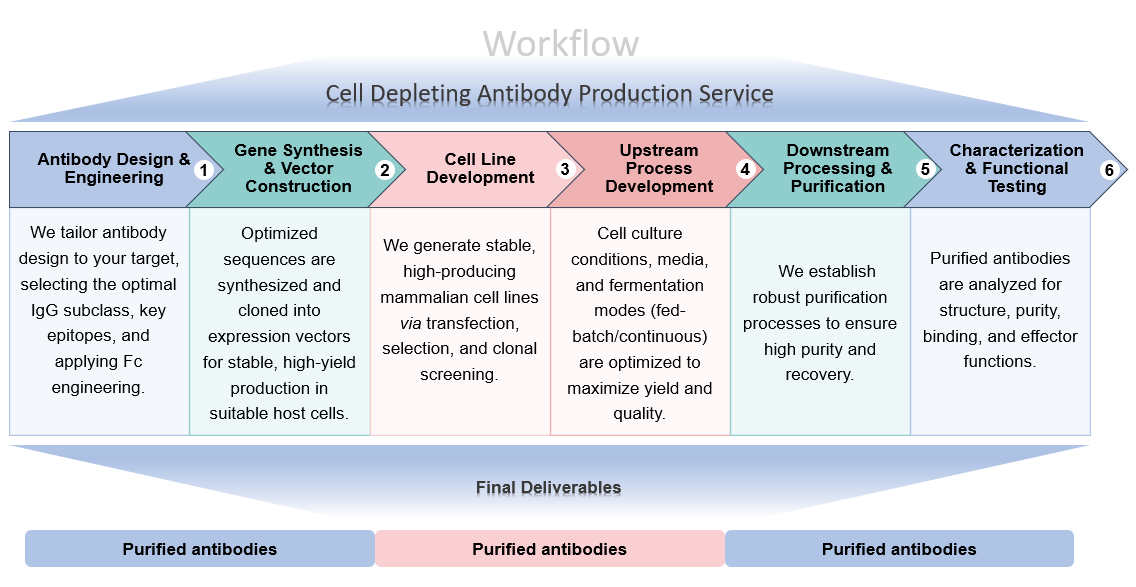
Standout Features
- Integrated End-to-End Solutions: We provide a seamless, one-stop service covering all stages from initial antibody design and engineering to large-scale production and comprehensive characterization.
- Scalable Production Capabilities: Our facilities are equipped with advanced bioreactors of varying volumes, from laboratory to pilot and large-scale industrial fermentation tanks, ensuring efficient and high-capacity production to meet your project's demands.
- Expert Upstream and Downstream Process Development: Our team excels in optimizing cell culture conditions, media, and purification strategies to maximize antibody yield, purity, and functional integrity.
- Advanced Fc Engineering Expertise: We specialize in both Fc protein-engineering and Fc glyco-engineering, allowing for precise modulation and enhancement of antibody effector functions.
- Rigorous Quality Management: Our well-established quality system, incorporating quality-by-design (QbD) principles and process analytical technologies (PAT), ensures consistent product quality and reproducibility.
- GMP-Compliant Production: We adhere to strict aseptic verification procedures throughout the production process and offer GMP-certified fermentation, ensuring the highest standards for therapeutic applications.
- Comprehensive Characterization: We employ high-standard quality control tools and a wide array of functional assays (ADCC, CDC, ADCP, apoptosis) to quantify and evaluate the quality and activity of your cell-depleting antibodies.
- Customized Service Models: We offer flexible engagement models, allowing for tailored optimization of codon usage, fermentation modes (batch, fed-batch, continuous), and culture conditions to maximize expression and yield for your specific antibody.
Representative Data
Summary: In this study, four types of CD19 antibodies were developed: the native form, a protein-engineered version (modified with EFTAE to enhance CDC), and afucosylated variants of both the native and protein-engineered types (to enhance ADCC). The results indicated that antibodies with the EFTAE modification exhibited superior CDC induction regardless of fucosylation status. Meanwhile, afucosylated antibodies showed enhanced ADCC activity. Notably, the doubly engineered antibody, which combined both the EFTAE modification and afucosylation, demonstrated significantly improved efficacy in triggering both CDC and ADCC.
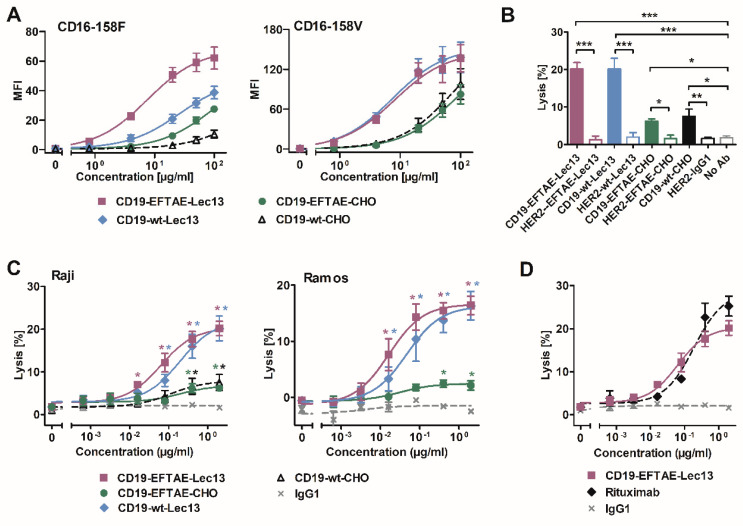 Fig.2 ADCC enhancement via Fc-modified CD19 antibodies targeting FcγRIIIA.2,3
Fig.2 ADCC enhancement via Fc-modified CD19 antibodies targeting FcγRIIIA.2,3
FAQs
How does Creative Biolabs ensure the high specificity and efficacy of cell-depleting antibodies?
We use advanced antibody engineering (precise Fc and glyco-engineering) plus rigorous functional assays (ADCC, CDC, ADCP, direct apoptosis) for optimal specificity and enhanced effector functions. Antibodies are designed to target specific cell surface antigens, maximizing therapeutic impact while minimizing off-target effects.
Can Creative Biolabs customize the effector functions of my antibody?
Yes. Our Fc engineering expertise lets us tailor effector mechanisms-enhanced ADCC via afucosylation, boosted CDC through protein modifications, or optimized direct apoptosis-creating antibodies with your required functional profile for therapeutic goals.
What is the typical turnaround time for a cell depleting antibody production project?
Timeframes vary by design complexity, production scale, and characterization needs. Our efficient workflows enable 12–24 week timelines. Discuss your project with our team for a precise estimate.
How does Creative Biolabs address potential safety concerns like immunogenicity or off-target effects?
Safety is prioritized via design-phase optimization: humanization strategies and careful Fc modifications reduce immunogenicity. Comprehensive characterization includes off-target binding assays to ensure a favorable safety profile.
Why Choose Us?
In a therapeutic landscape increasingly defined by precision and efficacy, the quality of your cell-depleting antibodies is paramount. Partner with Creative Biolabs to leverage our two decades of scientific expertise, state-of-the-art production facilities, and unwavering commitment to excellence. Let us be your trusted partner in advancing therapeutic frontiers and bringing life-changing treatments to patients worldwide.
Contact us for more information and to discuss your project.
References
- Mariottini, Alice et al. "Antibody-mediated cell depletion therapies in multiple sclerosis." Frontiers in immunology vol. 13 953649. 12 Sep. 2022. DOI: https://doi.org/10.3389/fimmu.2022.953649
- Roßkopf, Sophia et al. "Enhancing CDC and ADCC of CD19 Antibodies by Combining Fc Protein-Engineering with Fc Glyco-Engineering." Antibodies (Basel, Switzerland) vol. 9,4 63. 17 Nov. DOI: https://doi.org/10.3390/antib9040063
- Distributed under Open Access License CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

