Immune Checkpoint Blocking Antibody Production Service
Are you encountering difficulties in generating highly specific and potent immune checkpoint blocking antibodies, navigating complex manufacturing workflows, or looking for cutting-edge strategies in therapeutic antibody development? Our immune checkpoint blocking antibody production service streamlines your antibody discovery process, delivering high-quality, functionally characterized antibodies by leveraging advanced recombinant expression systems and state-of-the-art antibody engineering technologies.
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy by reactivating the body's immune system to target tumors. Antibodies blocking key immune checkpoints like PD-1, PD-L1, and CTLA-4 have shown remarkable clinical efficacy. However, challenges such as high production costs, scalability limitations, and the emergence of resistance necessitate innovative production platforms and a deeper understanding of regulatory mechanisms.
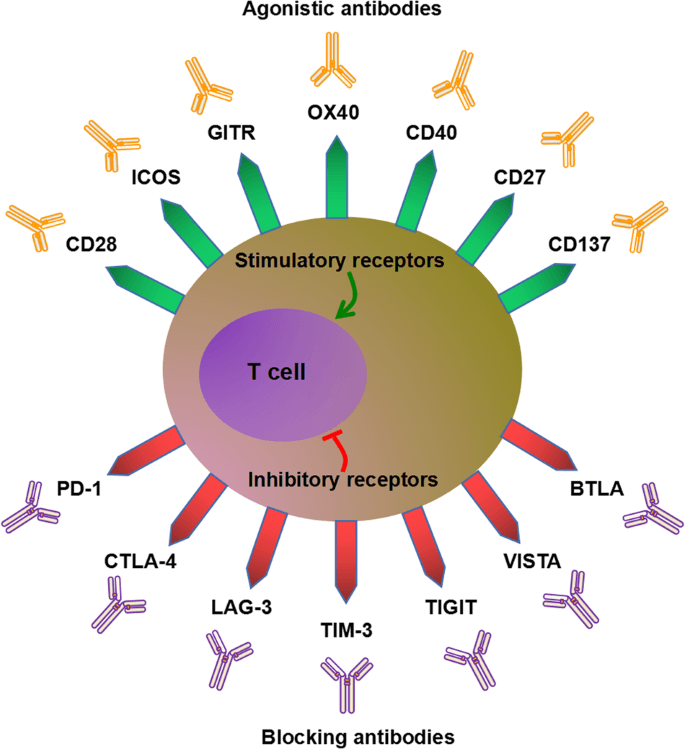 Fig.1 Agonist and blocking antibodies modulating immune checkpoints in tumor immunity.1,3
Fig.1 Agonist and blocking antibodies modulating immune checkpoints in tumor immunity.1,3
Our Services
Creative Biolabs addresses these needs by offering advanced production services, leveraging both established mammalian cell lines and cutting-edge plant-based systems, ensuring cost-effective, scalable, and functionally superior immune checkpoint blocking antibodies.
We offer a comprehensive immune checkpoint blocking antibody production service designed to deliver high-quality, functionally validated antibodies tailored to your specific research and therapeutic development needs. We provide end-to-end solutions, from gene synthesis and vector construction to large-scale antibody production and rigorous characterization, ensuring your project progresses efficiently and successfully. Our expertise in diverse production platforms allows us to overcome common challenges in antibody development, providing you with superior products for preclinical and clinical applications.
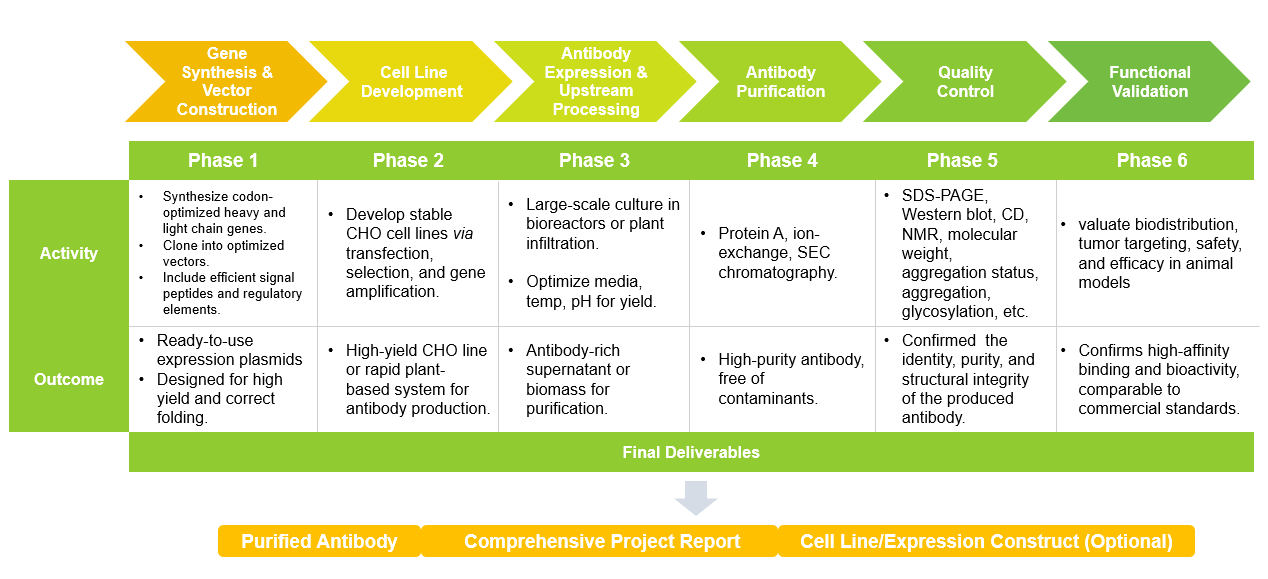
Workflow Procedures
Standout Features
Representative Data
Summary: This study aimed to develop a stable mammalian expression system for efficient anti-PD-1 monoclonal antibody production. Eight recombinant plasmids were constructed using two vectors (pOptiVEC and pcDNA3.3) with various signal peptides and transfected into CHO DG44 cells. Both vectors supported antibody expression, with signal peptides significantly affecting yield. Optimized culture conditions further improved production. The pO-SP4-HC/pD-SP3-LC combination achieved the highest titer (74.5 mg/L). The resulting antibodies showed appropriate molecular weight, low aggregation, and favorable physicochemical properties, supporting their potential in cancer immunotherapy.
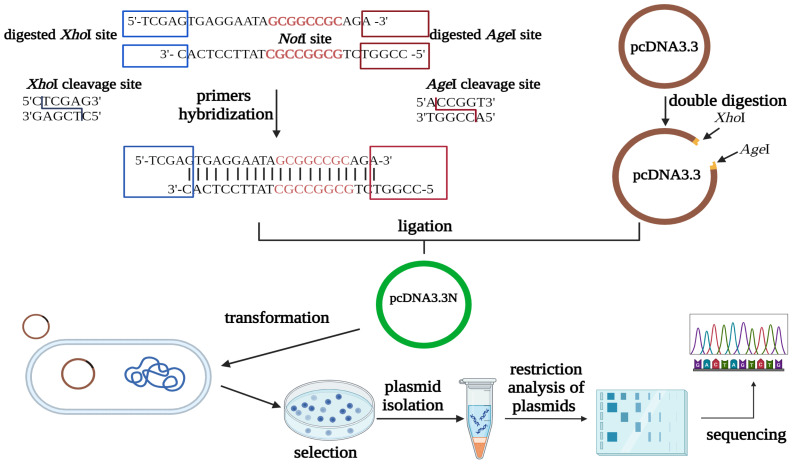 Fig.2 Plasmid construction strategy.2,3
Fig.2 Plasmid construction strategy.2,3
FAQs
What types of immune checkpoint blocking antibodies can Creative Biolabs produce?
We are equipped to produce a wide range of immune checkpoint blocking antibodies, including those targeting established checkpoints like PD-1, PD-L1, CTLA-4, LAG-3, and TIM-3, as well as emerging targets. We can handle various antibody isotypes and formats. If you have a specific target in mind, please don't hesitate to inquire!
How does Creative Biolabs ensure antibody quality and functionality?
Through multi-stage quality control, including structural analyses (SDS-PAGE, mass spectrometry, CD, NMR) and functional assays (ELISA, SPR, cell-based bioassays), we guarantee purity, integrity, and bioactivity.
Can Creative Biolabs assist with optimizing antibody design for improved expression or function?
Absolutely. Our team of experts can provide consultation on antibody design, including codon optimization, signal peptide selection, and other engineering strategies to enhance expression levels and functional properties. Our deep understanding of molecular mechanisms, including the impact of regulatory elements, allows us to optimize your antibody for peak performance.
Why Choose Us?
Creative Biolabs is your trusted partner for high-quality Immune Checkpoint Blocking Antibody Production. With our advanced platforms, rigorous quality control, and deep scientific expertise, we are dedicated to accelerating your journey from discovery to therapeutic success. We understand the complexities of modern immunotherapy and are equipped to provide the precise, high-performance antibodies your projects demand.
Customer Reviews:
"Immune checkpoint locking antibody production service in our research has significantly improved our ability to obtain high yields of complex IgG4 antibodies, which was a major bottleneck with our previous in-house mammalian systems. The purity was outstanding, and the functional data precisely matched our expectations, allowing us to move forward with our in vivo studies much faster. Their attention to N-glycosylation patterns was particularly beneficial, ensuring mammalian-like structures crucial for our therapeutic application." — 2024, A. J. S***th
"Creative Biolabs' rapid transient plant-based production for our anti-PD-L1 antibody was a game-changer. We needed quick, cost-effective material for early-stage screening, and their service delivered a functionally active antibody in just weeks. This speed, combined with the inherent safety advantages of plant platforms, provided a clear advantage over traditional methods and allowed us to explore more candidates efficiently." — 2023, Dr. K. M. W***g
"The insights gained from Creative Biolabs' comprehensive characterization of our immune checkpoint antibodies were invaluable. Their detailed analysis helped us understand how specific modifications could impact binding and T-cell activation, even hinting at potential resistance mechanisms. This deep mechanistic understanding is critical for designing next-generation immunotherapies that can overcome current limitations, especially concerning emerging regulatory molecules like circRNAs." — 2024, P. R. C***z
Contact us for more information and to discuss your project.
References
- Meng, Lingjiao et al. "Mechanisms of immune checkpoint inhibitors: insights into the regulation of circular RNAS involved in cancer hallmarks." Cell death & disease vol. 15,1 3. 4 Jan. 2024. DOI: https://doi.org/10.1038/s41419-023-06389-5
- Csató-Kovács, Erika et al. "Development of a Mammalian Cell Line for Stable Production of Anti-PD-1." Antibodies (Basel, Switzerland) vol. 13,4 82. 3 Oct. 2024. DOI: https://doi.org/10.3390/antib13040082
- Distributed under Open Access License CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

