Kidney Targeting Antibody-coupled Liposome Development Service
Kidney-targeting antibody-coupled liposomes offer a promising strategy for drug delivery and therapeutic applications, particularly for kidney conditions. Creative Biolabs specializes in liposomes harness antibodies or antibody fragments that selectively bind to receptors or antigens on renal or kidney cells, enabling the precise delivery of therapeutic agents directly to kidney tissues.
Introduction
Liposomes are spherical structures composed of phospholipids and cholesterol, which can be either naturally derived or synthetically produced. The phospholipid structure may bear a charge to facilitate control over its absorption and arrangement. Currently, the most commonly used products are unilamellar vesicles approximately 100 nm in size. At Creative Biolabs, our staff enhances the spatial hindrance of liposomes by incorporating polymers such as polyethylene glycol. This approach protects the loaded drugs from inactivation or metabolic degradation, thereby further increasing stability and improving cellular uptake. Furthermore, we combine liposomes with specific antibodies to achieve targeted effects, where passive targeting is primarily applied to cancer treatment, relying on the characteristics of tumor vasculature, and active targeting is achieved through functionalizing the liposome surface.
Creative Biolabs has developed a variety of techniques for liposome preparation, including the thin-film hydration method, ethanol/ether injection method, reverse evaporation method, detergent removal method, microfluidic method, membrane extrusion method, homogenization, ultrasonication, freeze-drying, and supercritical fluid methods. Additionally, we offer a range of characterization services for liposomes, such as particle size, zeta potential, morphology, phase behavior, and lamellarity.
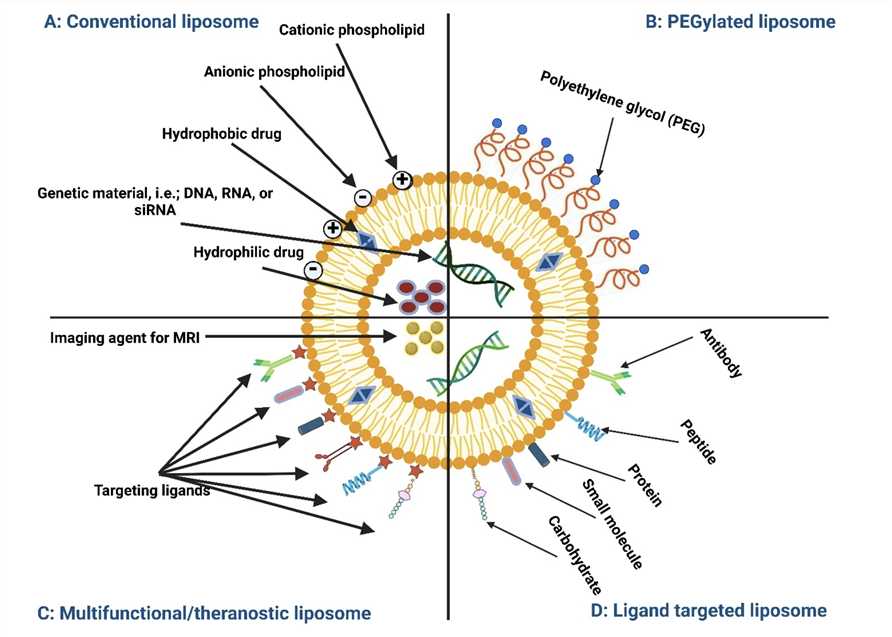 Fig.1 Liposome Types.1,3
Fig.1 Liposome Types.1,3
Services
Nowadays, Creative Biolabs offers a series of kidney-targeting antibody-coupled liposome production services that can be provided to researchers and pharmaceutical companies looking to develop targeted drug delivery systems.
Step1: Design and Formulation
Liposome Development: Create a range of liposome formulations with specific adjustments in size, lipid composition, and charge for optimal renal targeting.
Antibody Selection: Identify and evaluate antibodies that demonstrate a strong affinity for renal-specific antigens or receptors.
Step2: Conjugation of Antibodies to Liposomes
Linker Chemistry: Develop and refine linker chemistry to facilitate the efficient attachment of antibodies to liposomes while preserving their integrity and function.
Conjugation Techniques: Employ various methods, including click chemistry, PEGylation, and other bioconjugation strategies, to attach antibodies securely to the liposomal surface.
Step3: Characterization
Physicochemical Analysis: Evaluate particle size, polydispersity index (PDI), zeta potential, and encapsulation efficiency using techniques like Dynamic Light Scattering (DLS) and Transmission Electron Microscopy (TEM).
Stability Assessment: Investigate the long-term stability and shelf-life of antibody-conjugated liposomes under diverse storage conditions.
Step4: In Vitro Evaluation
Cell Culture Experiments: Perform studies using kidney cell lines to measure the binding affinity, uptake, and cytotoxicity of the antibody-conjugated liposomes.
Release Kinetics: Analyze the drug release profiles of the liposome formulations.
Step5: In Vivo Studies
Animal Model Development: Conduct pharmacokinetic and biodistribution studies in appropriate animal models to evaluate the efficacy of kidney targeting.
Efficacy Assessment: Investigate the therapeutic effectiveness in disease models, such as chronic kidney disease or cancer, utilizing the targeted liposomal formulations.
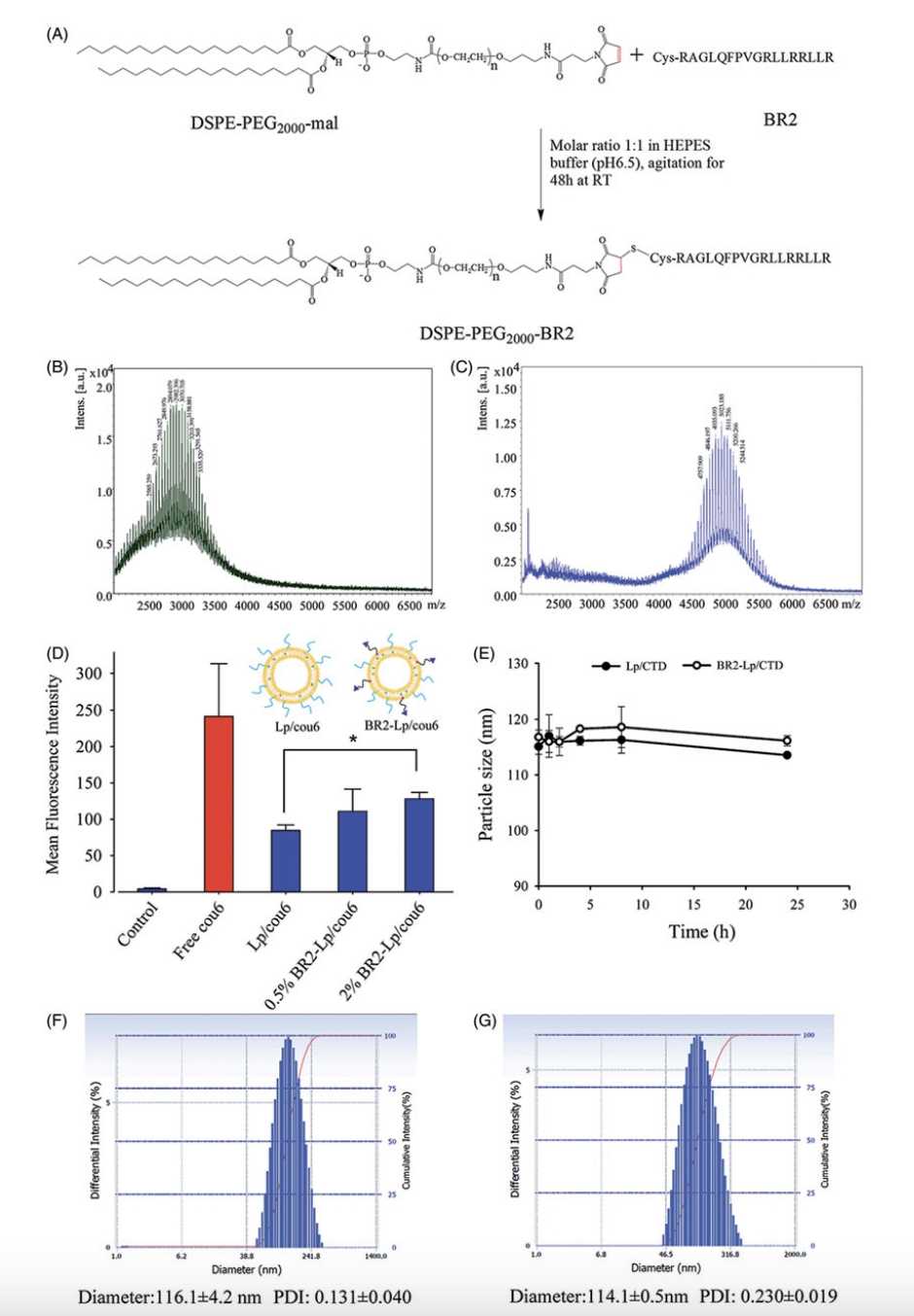 Fig.2 DSPE-PEG2000-BR2 Conjugation.2,3
Fig.2 DSPE-PEG2000-BR2 Conjugation.2,3
Case Study
In recent studies, antibody-coupled liposome conjugates have emerged as a promising solution for enhancing the specificity and effectiveness of drug delivery to kidney tissues. We have explored the methodology, production processes, and challenges associated with the development of kidney-targeting antibody-coupled liposome conjugates.
In our labs, liposomes were prepared using a thin-film hydration method. Antibodies are activated using NHS (N-hydroxysuccinimide) to introduce reactive ester groups. We have evaluated the targeting efficiency and therapeutic potential of the conjugates in vitro and in vivo. Meanwhile, we have also tested the effectiveness of the conjugates in preclinical models of kidney disease. Our kidney-targeting liposomes can deliver cytotoxic agents specifically to kidney cancer cells, improving treatment outcomes and reducing side effects. They hold the potential to aid in the development of dialysis fluids that incorporate targeted therapies designed to directly improve kidney function or assist in the regeneration of kidney tissues.
- Precision Targeting
- Enhanced Bioavailability
- Versatile Formulation
- Minimized Immune Response
- Potential for Combination Therapy
The area of targeted drug delivery has gained significant attention in recent years, particularly in enhancing the specificity and efficacy of therapies for renal diseases. By conjugating antibodies that selectively bind to kidney-specific antigens on the surface of renal cells to liposomes, Creative Biolabs can ensure that various agents are delivered precisely where they are needed most. We appreciate your interest! Please feel free to contact us with any questions you may have.
- Al-Jipouri, Ali, et al. "Liposomes or extracellular vesicles: a comprehensive comparison of both lipid bilayer vesicles for pulmonary drug delivery." Polymers 15.2 (2023): 318.
- Zhang, Xue, et al. "Liposomes equipped with cell-penetrating peptide BR2 enhances chemotherapeutic effects of cantharidin against hepatocellular carcinoma." Drug delivery 24.1 (2017): 986-998.
- Distributed under Open Access license CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



