Lung Targeting Antibody-coupled Liposome Development Service
The effective coupling of lung-targeted liposomes with antibodies is an important service platform offered by Creative Biolabs, primarily utilized for the development of targeted drug delivery systems. By conjugating antibodies with liposomes, we can achieve precise drug delivery to the lungs. Nowadays, we can provide customers with fast services of various scales in less than three weeks.
Services
Liposomes can encapsulate hydrophilic drugs within their aqueous core or load hydrophobic drugs into the hydrocarbon chain region of the lipid bilayer. As a crucial delivery vehicle, liposomes are used in the formulation of messenger RNA (mRNA) vaccines, playing a vital role in the safe delivery of mRNA, as well as in its rapid release and efficient expression within cells.
Creative Biolabs offers modification services for liposome surfaces to enhance their tissue targeting capabilities, allowing for flexible adjustments in circulation time and action sites. After more than a decade of development, our liposome preparation and antibody conjugation technologies have become fully mature. Furthermore, by coupling specific antibodies to lung-targeting liposomes, we can achieve precise identification and binding to target cells in the lungs, increasing drug concentration at the target site while reducing damage to healthy tissues.
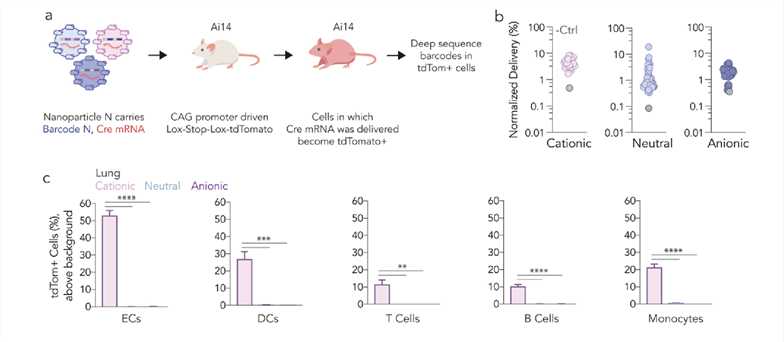 Fig.1 The Influence of Helper Lipid Charge on LNP In Vivo Tropism at the Cellular Level.1,3
Fig.1 The Influence of Helper Lipid Charge on LNP In Vivo Tropism at the Cellular Level.1,3
Process
As a leading company in the field of lung-targeting antibody-liposome conjugates, Creative Biolabs offers cost-effective, high-quality custom antibody-liposome production services using standard combinations of various lung-targeted liposomes, antibodies, and coupling methods. Our services include comprehensive and detailed analysis reports for the antibody-liposome conjugates. Creative Biolabs has prepared nearly a hundred antibody-liposome conjugates for clients using antibodies from diverse sources and of different isotypes, with production scales ranging from 1 milligram to 10 grams. These antibody-drug liposomes have been utilized in both in vitro and in vivo research.
A. Lipid Selection:
Choose appropriate lipids for liposome formulation, such as phospholipids and cholesterol. This selection should prioritize the desired liposome characteristics, including size, stability, and biocompatibility.
B. Liposome Formation:
Thin Film Hydration: Dissolve the lipids and subsequently evaporate them to create a thin film, which is then hydrated with a buffer to form liposomes.
Sonication or Extrusion: The resulting hydrated liposome solution may undergo sonication or extrusion to minimize size and achieve a uniform diameter.
C. Antibody Selection and Preparation:
Purify the specific monoclonal or polyclonal antibodies intended for attachment to the liposomes.
Characterize the antibodies to confirm their functionality and suitability for conjugation.
D. Conjugation:
Employ various techniques, such as passive adsorption or covalent coupling, to attach antibodies to the liposome surface.
Passive Adsorption: Mix antibodies with liposomes to allow for non-covalent binding, typically through ionic interactions.
Covalent Coupling: Utilize linker molecules to form covalent bonds between antibodies and reactive groups on the surface of the liposomes.
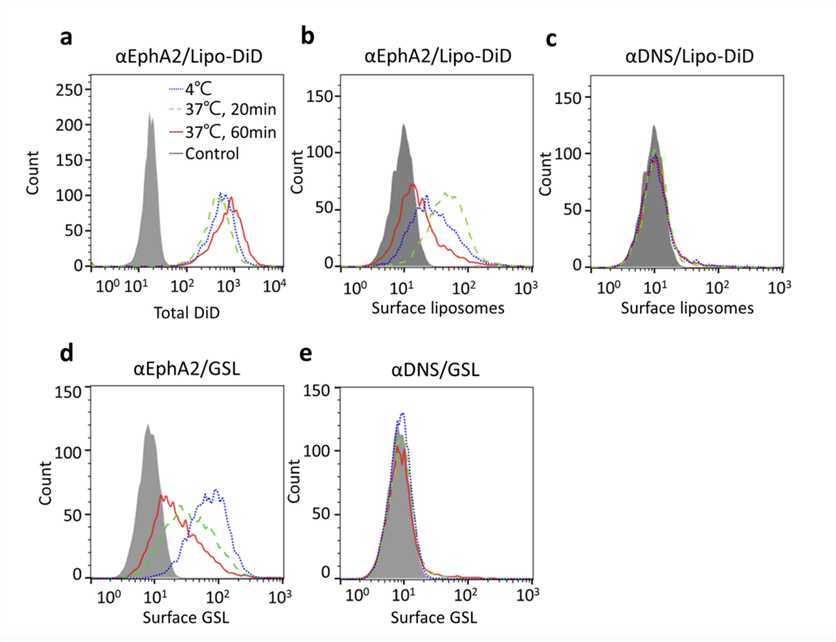 Fig.2 The Surface PEGylated Liposomal DiD Or GSL Was Measured Via Flow Cytometry.2,3
Fig.2 The Surface PEGylated Liposomal DiD Or GSL Was Measured Via Flow Cytometry.2,3
Case Study
In a recent project, we explored the design, production, characterization, and application of antibody-coupled liposomes aimed at enhancing drug delivery specifically to lung tissues.
Monoclonal antibodies targeting EphA2 and EGFR are selected for coupling. Liposomes consisting of DPPC and cholesterol have been designed to enhance stability. Antibodies are conjugated to liposomes through thiol-maleimide coupling and EDC/NHS chemistry, which help maintain the structural integrity and biological activity of the antibodies. DLS has been employed to assess the size distribution and stability of the liposomes. Additionally, transmission electron microscopy (TEM) offered valuable information regarding the structure and morphology of the liposomes. To quantify the amount of antibody attached to the liposome surface, ELISA techniques have been employed in our labs. Our liposomes can direct delivery drug agents precisely to the target site, minimizing off-target effects and reducing systemic toxicity, offering the potential for more effective treatments that are customized to the specific characteristics of lung diseases.
The design and construction of antibody-coupled liposomes hold significant promise in many disease therapies. Creative Biolabs is dedicated to developing customized liver-targeting antibody-coupled liposome strategies that align with your specific antigens. Our service harnesses the benefits of liposomes-low toxicity and the capacity to encapsulate various therapeutic agents alongside the specificity and targeting abilities offered by antibodies. Customer satisfaction is our priority. Feel free to contact us for a quote or place an order anytime!
- Radmand, Afsane, et al. "The transcriptional response to lung-targeting lipid nanoparticles in vivo." Nano letters 23.3 (2023): 993-1002.
- Ho, Kai-Wen, et al. "Targeted internalization and activation of glycosidic switch liposomes by an EphA2 PEG engager increases therapeutic efficacy against lung cancer." (2024).
- Distributed under Open Access license CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



