Antibody-PEGylated Liposome Production Service
Creative Biolabs is thrilled to introduce our production services for antibody-PEGylated liposomes, aimed at enhancing the precision and targeting of your drug delivery systems. Our team works hand-in-hand with you to create tailored antibody-pEGylated liposomes that align with your unique requirements, ensuring optimal targeting and release profiles.
Services
In recent years, antibody drugs have made significant progress in the treatment of human diseases. However, they still face several challenges, including short half-lives, high immunogenicity, low solubility, and susceptibility to enzymatic degradation. PEG modification can considerably extend the half-life of antibodies, enhance their pharmacokinetic properties, and reduce immunogenicity.
Creative Biolabs offers a range of PEG modification services for various antibody proteins, allowing for customization based on specific client needs. Key modification sites include the N-terminus and C-terminus of peptides, as well as the side chains of lysine (Lys) and the thiol groups of cysteine (Cys). Depending on requirements, CBL provides PEG monomers with molecular weights ranging from PEG2 to PEG24, while the molecular weight range for the macromolecular PEG used for modification spans from PEG500 to PEG40K. These options provide flexible solutions for clients to optimize the performance of their antibody drugs.
- High Efficiency: Utilizing highly effective PEGylation reagents and streamlined processes to ensure optimal modification efficiency and yield.
- High Quality: Rigorously controlling every aspect of the modification process to guarantee the purity and integrity of the final product.
- Customization: Offering tailored modification solutions and technical support based on the specific experimental needs and modification requirements of our clients.
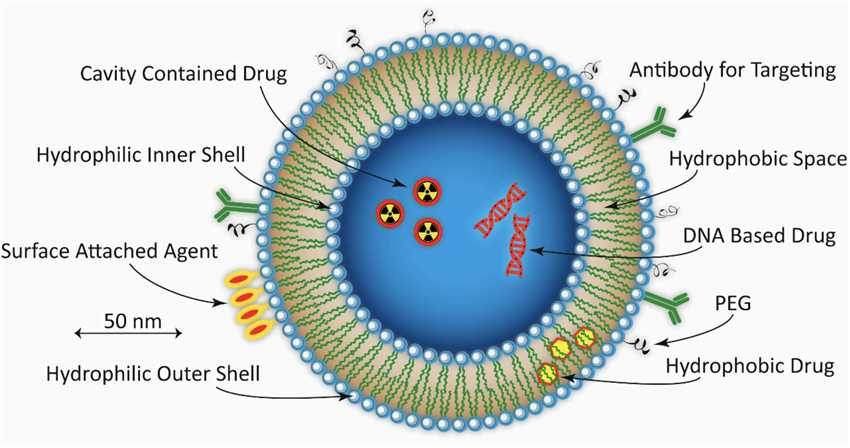 Fig.1 The Example of a Spherical PEGylated Liposome.1,3
Fig.1 The Example of a Spherical PEGylated Liposome.1,3
Procedures
The production of antibody-PEGylated liposomes involves several key steps that combine the principles of liposome formulation, PEGylation, and antibody conjugation.
- Step 1: Liposome Preparation
Select Lipid Composition: Choose suitable phospholipids and additional components, such as cholesterol, to construct the liposomal bilayer.
Hydrate Lipids: Dissolve the chosen lipids in an organic solvent (chloroform). Evaporate the solvent to yield a thin lipid film, then hydrate this film with an aqueous buffer (PBS) to generate multilamellar vesicles (MLVs).
Reduce Size: Employ methods such as sonication, extrusion, or microfluidization to decrease the size of the liposomes, resulting in uniform, small unilamellar vesicles (SUVs).
- Step 2: PEGylation of Liposomes
PEG Lipid Preparation: Utilize a lipid derivative incorporating a PEG moiety. Common options include DSPE-PEG and PEG-cholesterol.
Incorporation of PEG Lipids: Introduce the PEG-lipid during the liposome formulation process, or mix it with pre-formed liposomes to achieve surface PEGylation. This can be accomplished through passive loading, allowing the PEG-lipid to integrate into the lipid bilayer.
- Step 3: Antibody-PEGylation Conjugation
Antibody Selection: Select the appropriate monoclonal or polyclonal antibody for targeted delivery.
Conjugation Strategies:
Passive Loading: Incubate liposomes with antibodies in a specific buffer to facilitate their adsorption onto the liposome surface.
Covalent Conjugation: Employ crosslinking agents (e.g., EDC/NHS or maleimide-based methods) to covalently attach antibodies to the PEG chains on the liposome surface.
Purification: Purify the antibody-liposome conjugates by using a series of methods, including affinity chromatography.
- Step 4: Antibody-PEGylated Liposomes Characterization
Size and Zeta Potential Analysis: Dynamic light scattering (DLS) is used to measure the hydrodynamic size of liposomes and evaluate their polydispersity index (PDI), which indicates size uniformity. Zeta potential measurements further elucidate the surface charge of the liposomes, impacting their stability and interaction with biological tissues.
Morphological Assessment: Transmission electron microscopy (TEM) or scanning electron microscopy (SEM) can be employed to visualize the morphology of liposomes, allowing researchers to confirm their uniformity and structural integrity.
Antibody Integrity and Functionality Tests: The functionality of conjugated antibodies must be assessed to ensure they retain their activity post-conjugation.
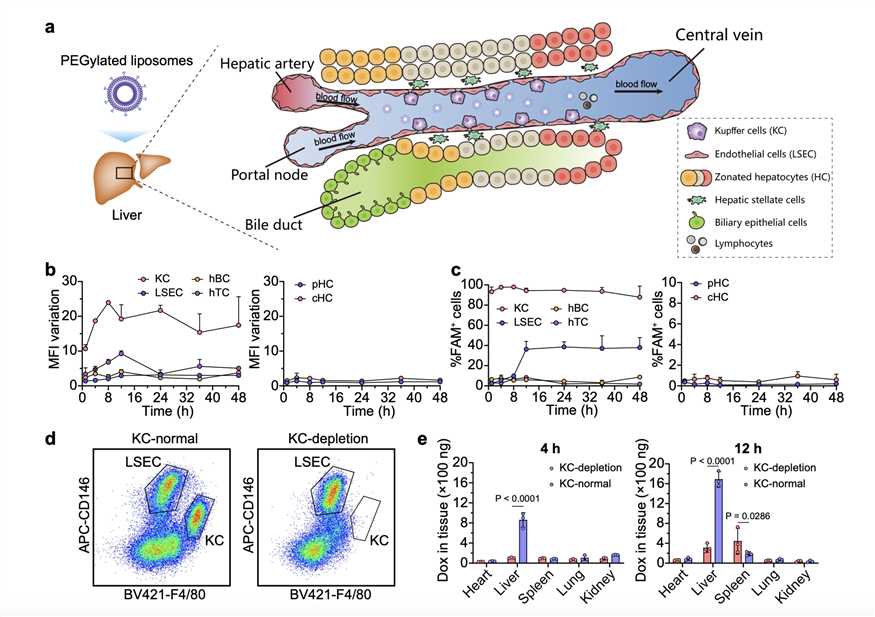 Fig.2 Intravenously Injected PEGylated Liposomes Interact With Various Hepatic Cells.2,3
Fig.2 Intravenously Injected PEGylated Liposomes Interact With Various Hepatic Cells.2,3
Applications
At Creative Biolabs, our antibody-PEGylated liposomes have been used in powerful drug delivery systems that merge the stability and biocompatibility of liposomes with the precise targeting capabilities of antibodies. Below are several key applications of antibody-PEGylated liposomes:
- Immunotherapy: Antibody-PEGylated liposomes can facilitate the targeted delivery of immune-modulating agents, such as checkpoint inhibitors or cytokines, to designated immune cells or tumor sites, thereby strengthening the body's immune response against cancer.
- Vaccine Delivery: Our liposomes can enhance the efficiency of delivering antigens or adjuvants, promoting a stronger immune response by ensuring targeted delivery to antigen-presenting cells, which ultimately increases vaccine efficacy.
- Inflammatory Diseases: In conditions such as rheumatoid arthritis or inflammatory bowel disease, antibody-PEGylated liposomes can specifically target inflamed tissues, enabling the direct delivery of anti-inflammatory drugs to the site of action.
- Central Nervous System Delivery: PEGylated liposomes can improve the transport of therapeutic agents across the blood-brain barrier, potentially aiding in the treatment of neurological disorders such as Alzheimer's disease or multiple sclerosis when paired with brain-targeting antibodies.
- Gene Therapy: Our products can be engineered to deliver nucleic acids, including siRNA or plasmid DNA, directly to specific cells or tissues via antibody-mediated targeting, thereby facilitating gene silencing or gene replacement therapies.
Creative Biolabs is at the forefront of antibody-PEGylated liposome production. With extensive expertise in biochemistry and pharmacology, we conduct comprehensive testing and characterization of all our products to ensure they adhere to the highest regulatory standards. Please feel free to contact us for any inquiries.
- Elgqvist, Jörgen. "Nanoparticles as theranostic vehicles in experimental and clinical applications-focus on prostate and breast cancer." International journal of molecular sciences 18.5 (2017): 1102.
- Jiang, Kuan, et al. "Kupffer cells determine intrahepatic traffic of PEGylated liposomal doxorubicin." Nature Communications 15.1 (2024): 6136.
- Distributed under Open Access license CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



