Lysine & Cysteine Conjugation mediated Antibody Bioconjugation Production Service
Are you encountering issues like vast conjugate heterogeneity, inconsistent reproducibility, or challenges in maintaining both stability and functional integrity of your antibody–drug (or antibody–label) conjugates? Our lysine & cysteine conjugation-mediated antibody bioconjugation production service helps you accelerate drug discovery and obtain high-quality, homogeneous, and stable antibody conjugates through advanced and site-selective bioconjugation techniques.
Introduction of Lysine & Cysteine Conjugation mediated Antibody Bioconjugation Production
Antibody bioconjugation, the chemical linkage of antibodies to various functional molecules, is pivotal in modern biotechnology, enabling targeted therapies, advanced diagnostics, and sophisticated research tools. Traditional methods, often relying on random lysine conjugation, frequently yield heterogeneous products with unpredictable properties and compromised functionality. In contrast, cysteine-mediated conjugation via site-selective disulfide reduction yields more uniform modifications. The cysteine-to-lysine transfer (CLT) strategy builds on this by using disulfide bonds to guide acylating agents to nearby lysines, combining amide bond stability with improved site-selectivity. CLT is compatible with native antibody fragments, avoids genetic engineering, and offers strong potential for generating uniform, functional bioconjugates.
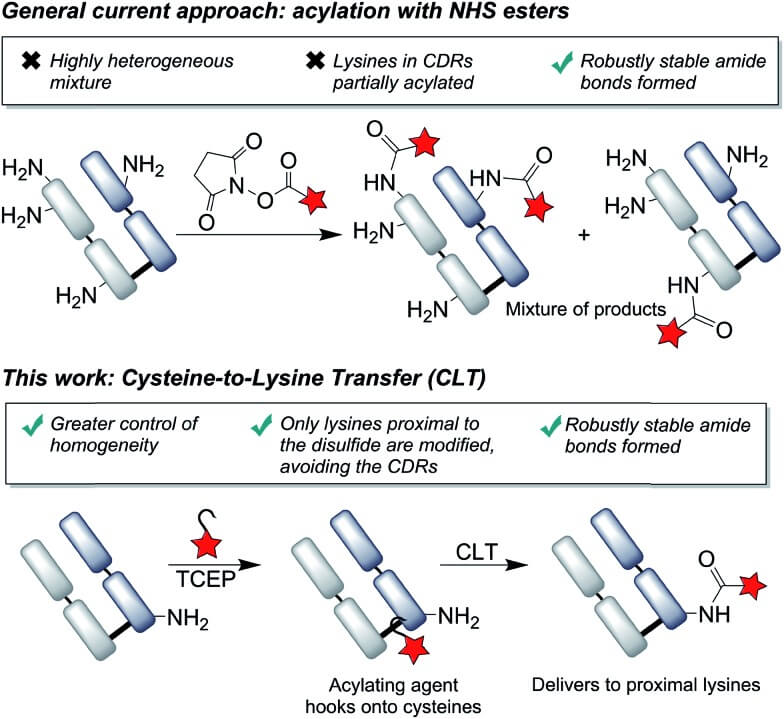 Fig.1 Proximal cysteines enable targeted lysine acylation in antibody fragments.1
Fig.1 Proximal cysteines enable targeted lysine acylation in antibody fragments.1
Lysine & Cysteine Conjugation mediated Antibody Bioconjugation Production Service at Creative Biolabs
Creative Biolabs delivers tailored solutions for the precise and efficient conjugation of antibodies with various payloads, addressing the critical need for well-defined bioconjugates in research, diagnostics, and therapeutics. Our service provides highly characterized antibody-drug conjugates, antibody-fluorophore conjugates, and other bespoke constructs, overcoming the limitations of traditional random conjugation. We ensure batch-to-batch consistency and optimal functional integrity, which are crucial for achieving preclinical and clinical success. Our expertise minimizes the risk of altered antigen binding or compromised in vivo properties, often associated with less controlled methods.
Creative Biolabs addresses limitations by offering specialized services utilizing both lysine and advanced cysteine-mediated conjugation strategies, including innovative site-specific approaches like CLT. Our methodologies ensure the production of homogeneous, stable, and functionally optimized antibody conjugates essential for reliable and impactful results in drug discovery and development.
Our Service Process
Our comprehensive workflow is designed for transparency and efficiency, ensuring high-quality outputs at every stage.
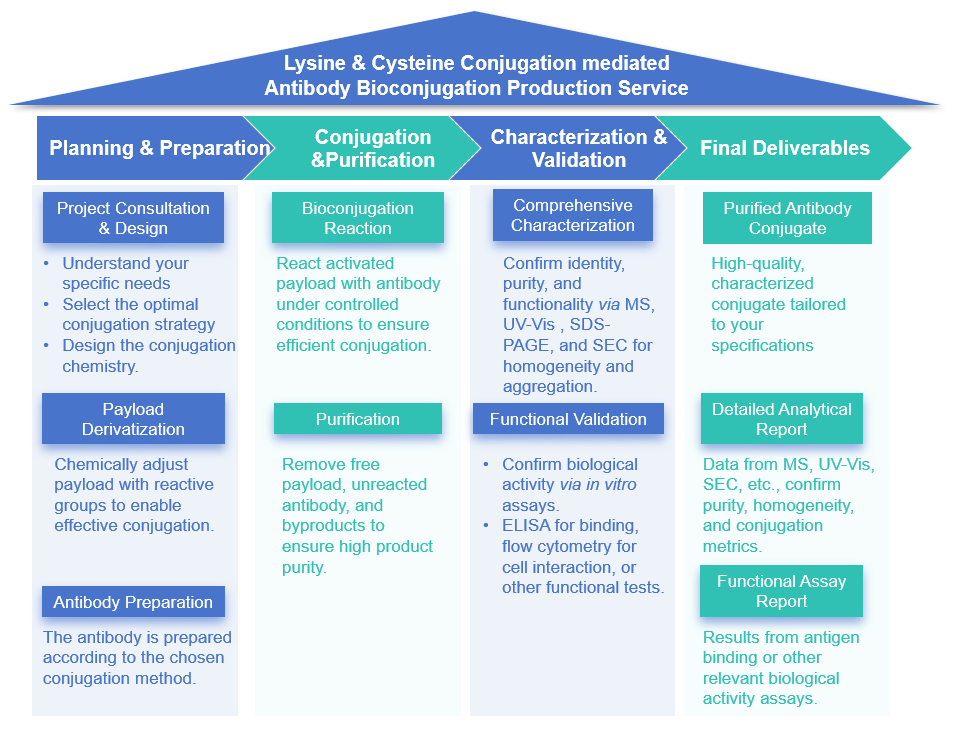
Core Strengths
- Precision Site-Specific Conjugation: Overcome the limitations of traditional random labeling by leveraging advanced methodologies like second-generation maleimides and our proprietary CLT technology, ensuring highly homogeneous conjugates with predictable properties.
- Customized Conjugation Strategies: Tailored project design based on your specific antibody, payload, and application requirements, optimizing conjugation chemistry for ideal fluorophore-to-antibody ratio or drug-to-antibody ratio and functional outcomes.
- Comprehensive Analytical Validation: Rigorous characterization using state-of-the-art mass spectrometry (including LC-MS/MS for site identification), UV-Vis spectroscopy, SDS-PAGE, and size-exclusion chromatography to guarantee the purity, homogeneity, and structural integrity of every conjugate.
- Enhanced Conjugate Stability & Functionality: Production of conjugates with superior long-term stability under various storage and environmental conditions, while meticulously preserving the antibody's antigen binding affinity and biological activity, critical for reliable in vitro and in vivo performance.
- Expert Consultation & Support: Direct access to our team of seasoned biology and chemistry experts who will guide you through every stage of your project, from initial concept to final delivery, ensuring scientific excellence and successful results.
- Scalable Production Capabilities: From small-scale research batches to larger-scale production, our flexible platforms are designed to meet your project's evolving needs while maintaining consistent quality and reproducibility.
Data Supporting
Summary: Lysine-based antibody conjugation forms stable amide bonds but often yields heterogeneous products. This study introduces a CLT strategy, using native disulfide bonds to guide acylating agents to specific lysines away from the binding site. Applied to trastuzumab Fab fragments, CLT enables site-selective, stable conjugation without genetic modification. Both mono- and bis-thioesters produced homogeneous conjugates that retained antigen-binding activity. CLT offers a robust, reproducible method for next-generation antibody conjugates.
Results: The CLT strategy was applied to trastuzumab Fab fragments with one disulfide bond and 26 lysines. MESNa thioester enabled selective acyl transfer to K136, K221, and K225 under optimized conditions. Conjugates showed stability, no aggregation, preserved binding, and efficient fluorophore labeling. A bis-thioester improved regioselectivity, yielding a single-acylated product at K136. Both strategies produced homogeneous, functional antibody conjugates.
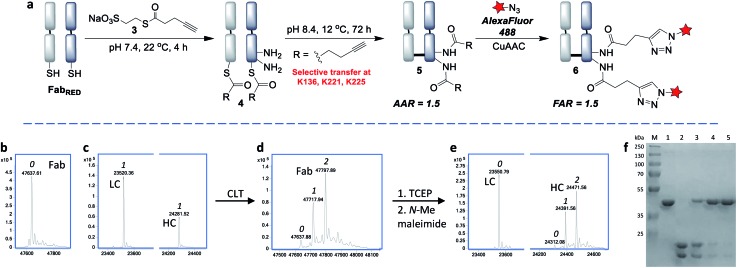 Fig.2 Cysteine-to-lysine transfer (CLT) strategy using MESNa thioester 3.1
Fig.2 Cysteine-to-lysine transfer (CLT) strategy using MESNa thioester 3.1
FAQs
Why is conjugate homogeneity important, and how does Creative Biolabs achieve it?
Homogeneity ensures consistent bioactivity, predictable pharmacokinetics, and low immunogenicity—vital for therapeutics. Random lysine conjugation often causes heterogeneity. We overcome this using site-specific methods such as interchain cysteine cross-linking with second-generation maleimides and our proprietary CLT technology. These enable precise control over payload number and location.
Do your conjugation methods affect antigen binding?
Preserving antigen binding is essential. Our site-specific strategies, especially CLT, target regions away from CDRs to avoid interference. We validate binding through ELISA, flow cytometry, and other functional assays to ensure activity is retained.
What are the benefits of cysteine-mediated over lysine conjugation?
Lysine conjugation is simple but often produces heterogeneous products. Cysteine-based methods, particularly with SGMs, enable more precise and reproducible conjugates by targeting disulfide bonds. Our CLT approach further combines site-specificity with stable, amide-linked conjugates.
How do you ensure conjugate stability?
We use robust linkers and chemistries known for stability. Each conjugate undergoes thorough testing under varied storage and pH conditions. Our SGM-based conjugates, for example, show superior stability over conventional methods.
Can your service handle antibody fragments?
Yes. We support full antibodies and fragments (e.g., Fab, scFv). Our methods are well-suited to fragments, offering precise conjugation while preserving their advantages, like better tumor penetration and customizable half-life.
Why Choose Us?
Creative Biolabs is your trusted partner for high-quality, homogeneous, and stable antibody bioconjugates. By leveraging our expertise in both lysine and advanced cysteine-mediated conjugation, including innovative site-specific approaches, we empower your research and accelerate your therapeutic development.
Contact us today to explore your project and discover how our services can support you in achieving your research objectives.
Reference
- Forte, Nafsika et al. "Cysteine-to-lysine transfer antibody fragment conjugation." Chemical science vol. 10,47 10919-10924. 11 Oct. 2019. Distributed under Open Access License CC BY 4.0, without modification. DOI: https://doi.org/10.1039/C9SC03825F
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



