Controlled Release Kinetic Evaluation Services
Are you currently facing unpredictable drug performance, challenges in optimizing your drug delivery systems, or concerns about off-target toxicity? Our controlled release kinetic evaluation services help you accelerate drug development, ensure precise drug performance, and minimize side effects through advanced analytical methodologies and comprehensive kinetic modeling.
Introduction
Controlled release technology has revolutionized the delivery of active agents, offering unprecedented control over their therapeutic effects. By precisely governing the rate, duration, and location of active substance release, these systems enhance drug efficacy, improve patient safety, and increase compliance. The meticulous evaluation of release kinetics is paramount to realizing these benefits, providing the foundational understanding necessary for successful product development.
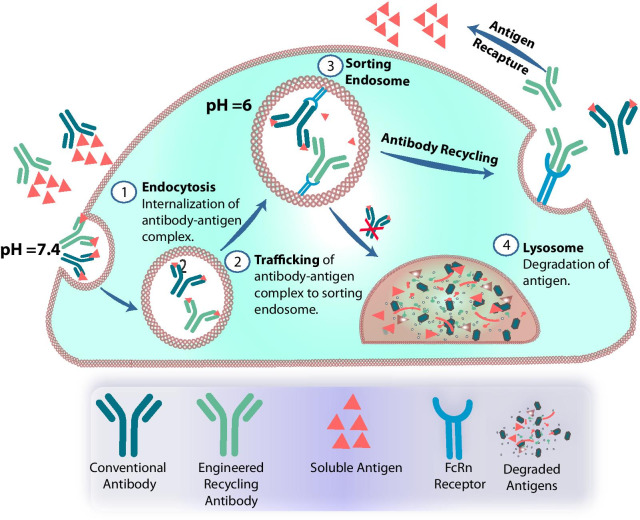 Fig.1 Schematic diagram of environment-responsive antibodies.1,3
Fig.1 Schematic diagram of environment-responsive antibodies.1,3
Controlled Release Kinetic Evaluation Services at Creative Biolabs
At Creative Biolabs, our controlled release kinetic evaluation services are designed to provide you with a profound understanding of how your active ingredients are released from their delivery systems. We conduct comprehensive studies to characterize the rate and extent of payload release under simulated physiological and tumor-specific conditions (e.g., varying pH, enzyme concentrations). Additionally, we evaluate the stability of the antibody-linker-payload construct in circulation versus its specific cleavage and release at the target site. These assessments yield quantifiable release profiles, a clear mechanistic understanding of drug release, and optimized formulation parameters. Our data is meticulously prepared to support your regulatory submissions and provides critical insights for predicting in vivo behavior, ultimately de-risking your development pipeline.
How It Works
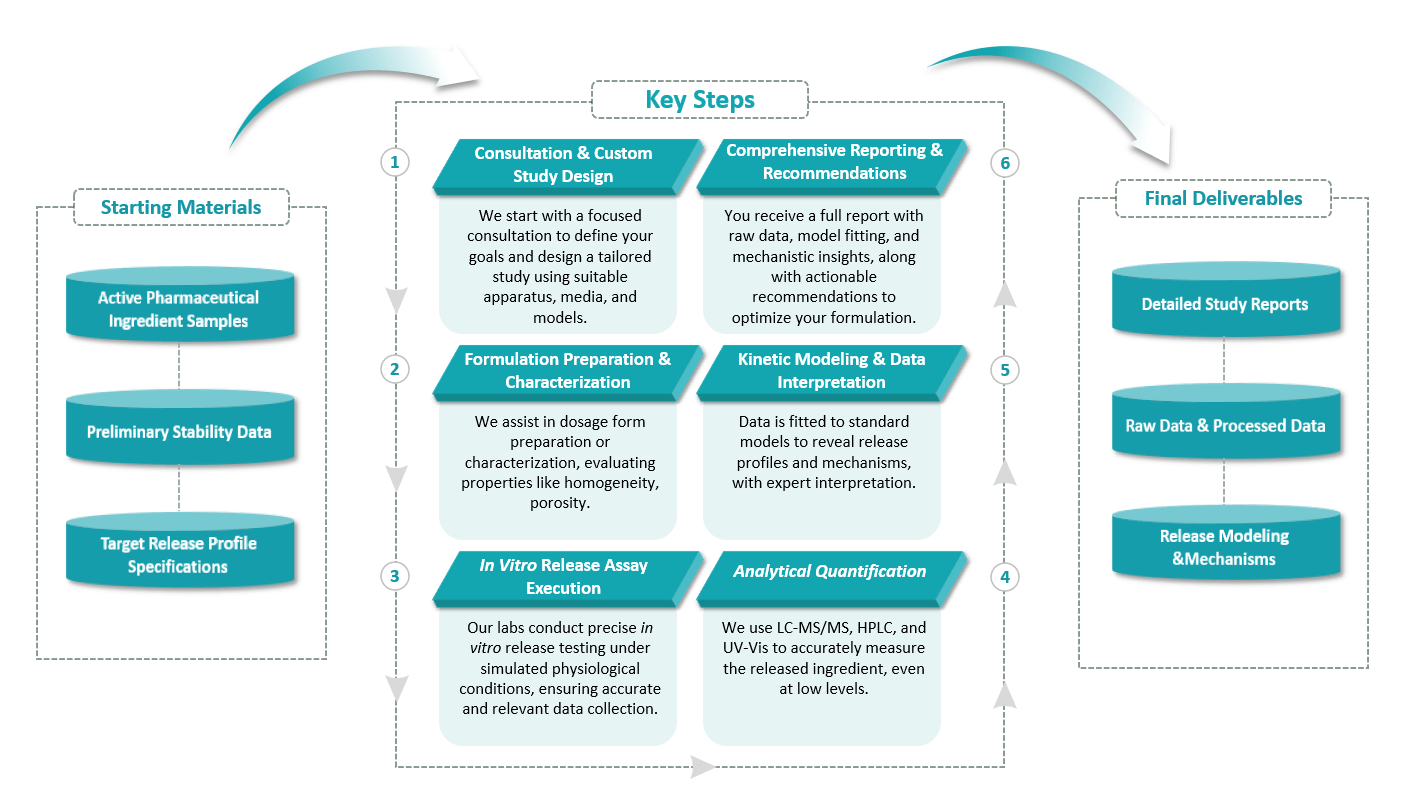
Types of our Controlled Release Kinetic Evaluation Services
In Vitro Release Assays
These assays are the cornerstone of our services, designed to simulate physiological and pathological conditions while quantifying the release kinetics of your active ingredient. pH-dependent release studies evaluate how formulations respond across a range of pH values, mimicking gastrointestinal transitions or the acidic conditions of specific tissues such as tumors. Enzyme-mediated release studies utilize biologically relevant enzymes, or tissue-specific proteases-to trigger and quantify payload release from cleavable linkers or biodegradable matrices. To provide a physiologically relevant context, we also conduct simulated biological fluid studies in complex media such as plasma, interstitial fluid, or tumor microenvironment analogs. Together, these assays provide actionable insights into formulation behavior, enabling data-driven design and optimization of controlled release systems.
Responsive Construct Stability Assessment
We rigorously assess the structural and functional stability of antibody-linker-payload constructs under physiological conditions, ensuring their integrity during circulation and their precise activation at the target site. This includes evaluating release kinetics in response to a variety of biologically relevant stimuli. Redox potential studies simulate the intracellular reducing environment, particularly relevant for disulfide-linked conjugates as commonly employed in ADC designs. Temperature-triggered assessments examine the behavior of thermo-responsive polymers and formulations under controlled thermal shifts. Photo-activation studies are used to evaluate constructs designed for light-triggered payload release. Additionally, we assess responsiveness to specific biomarkers, such as enzymes or pathological pH gradients characteristic of disease sites like the tumor microenvironment. These comprehensive studies help de-risk your formulation strategy and guide rational design of next-generation therapeutics.
Case Study
Summary: This study developed a tumor environment-responsive platform, combining two small antibodies: one targets a surface protein (PD-L1) on cancer cells, the other a cancer-related protein inside. It includes a sequence cut by a specific enzyme and protein-degrading sequences. Lab tests showed it breaks down the intracellular target protein via the body's protein breakdown system, causing cancer cell death and stopping bladder cancer cells from growing/spreading. Animal tests confirmed strong anti-tumor effects with low toxicity, reducing the target protein and inhibiting tumor growth. In conclusion, this platform precisely breaks down harmful intracellular proteins in cancer cells, boosting treatment efficacy and offering a new targeted cancer therapy approach.
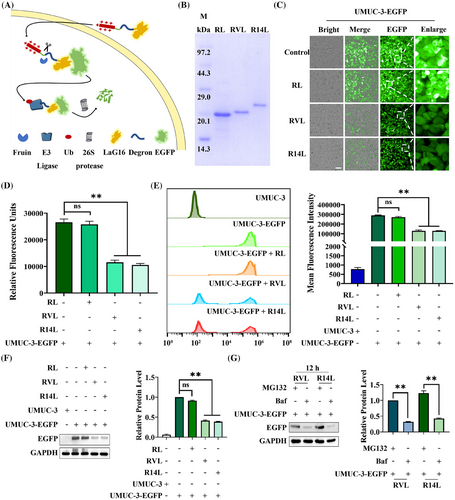 Fig.2 Establishment and validation of an antibody-mediated targeted degradation platform.2,3
Fig.2 Establishment and validation of an antibody-mediated targeted degradation platform.2,3
FAQs
How do your services compare to in-house testing?
Partnering with Creative Biolabs provides access to specialized expertise, advanced instrumentation, and validated methodologies that might be costly or time-consuming to establish in-house. Our services can accelerate your development timelines, reduce your operational overhead, and provide robust, regulatory-ready data, allowing your team to focus on core research and development.
How do you ensure the accuracy of your kinetic data?
Our commitment to accuracy is paramount. We employ state-of-the-art analytical instrumentation, including highly sensitive LC-MS/MS, and adhere to rigorous quality control standards. Our methodologies are optimized through extensive research, and we utilize orthogonal analytical techniques to cross-validate results, ensuring the highest level of precision and reliability.
Can you help us understand the release mechanism of our novel delivery system?
Absolutely. Beyond simply providing release profiles, our service includes comprehensive kinetic modeling and expert data interpretation. We apply various mathematical models to elucidate the underlying release mechanisms, such as diffusion, erosion, or swelling, providing critical insights for your formulation development.
Why Choose Us?
Creative Biolabs is your trusted partner for navigating the complexities of controlled release. Our unparalleled expertise, cutting-edge analytical capabilities, and commitment to delivering actionable insights empower you to develop superior products with optimized performance and enhanced patient benefits.
Customer Reviews:
"Creative Biolabs helped us understand the exact enzymatic cleavage kinetics of our novel prodrug, revealing unexpected pH dependencies. Their detailed kinetic modeling and expert interpretation facilitated a crucial redesign of our linker, avoiding potential in vivo stability issues." – 2023, S***h Johnson
"The comprehensive reports and clear recommendations from Creative Biolabs' kinetic evaluation team dramatically accelerated our formulation optimization. Their ability to correlate in vitro release with predicted in vivo behavior saved us months of experimental work compared to our previous approaches." – 2024, M***a Rodriguez
How to Contact Us
Ready to advance your controlled release project? Our team of experts is eager to discuss your specific needs and provide tailored solutions.
References
- Klaus, Tomasz, and Sameer Deshmukh. "pH-responsive antibodies for therapeutic applications." Journal of biomedical science vol. 28,1 11. 22 Jan. 2021. DOI: https://doi.org/10.1186/s12929-021-00709-7
- Deng, Changping et al. "Targeting intracellular cancer proteins with tumor-microenvironment-responsive bispecific nanobody-PROTACs for enhanced therapeutic efficacy." MedComm vol. 6,2 e70068. 19 Jan. 2025. DOI: https://doi.org/10.1002/mco2.70068
- Distributed under Open Access License CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

