Responsive Construct Stability Assessment Service
Are you facing challenges in ensuring the stability and targeted release of your advanced drug candidates? Creative Biolabs' responsive construct stability assessment service helps you accelerate drug development and obtain high-quality, precisely characterized therapeutics through advanced bioanalytical and modeling techniques. We provide comprehensive solutions for evaluating and optimizing the stability and controlled release of complex therapeutic agents.
Introduction
Responsive construct stability assessment service focuses on the critical balance between maintaining the integrity of advanced drug delivery systems in circulation and ensuring their precise, triggered release at the target site. This service is vital for Antibody-Drug Conjugates (ADCs) and smart nanoparticles, where systemic stability prevents off-target toxicity, and controlled release maximizes therapeutic efficacy.
Responsive Construct Stability Assessment Service at Creative Biolabs
At Creative Biolabs, our responsive construct stability assessment service delivers precise, data-driven solutions to optimize the stability and targeted release of your drug constructs. We rigorously evaluate the stability of the antibody–linker–payload construct in circulation versus its specific cleavage and release at the target site, ensuring the payload remains intact during systemic circulation and is efficiently delivered within pathological tissues. Leveraging advanced analytical platforms, our service provides detailed insights into how your ADCs or smart nanoparticles behave within complex biological environments, including assessments of pH stability, thermal stability, aggregation propensity, and in vitro/in vivo plasma stability.
Furthermore, we design experiments tailored to simulate physiologically and pathologically relevant stimuli—such as acidic conditions found in tumor microenvironments or lysosomes-to validate controlled payload release upon trigger activation. By closely analyzing linker chemistry, conjugation sites, and drug-antibody ratio (DAR), our assessments help minimize off‑target toxicities, enhance therapeutic efficacy, and streamline your development pipeline.
Service Workflow
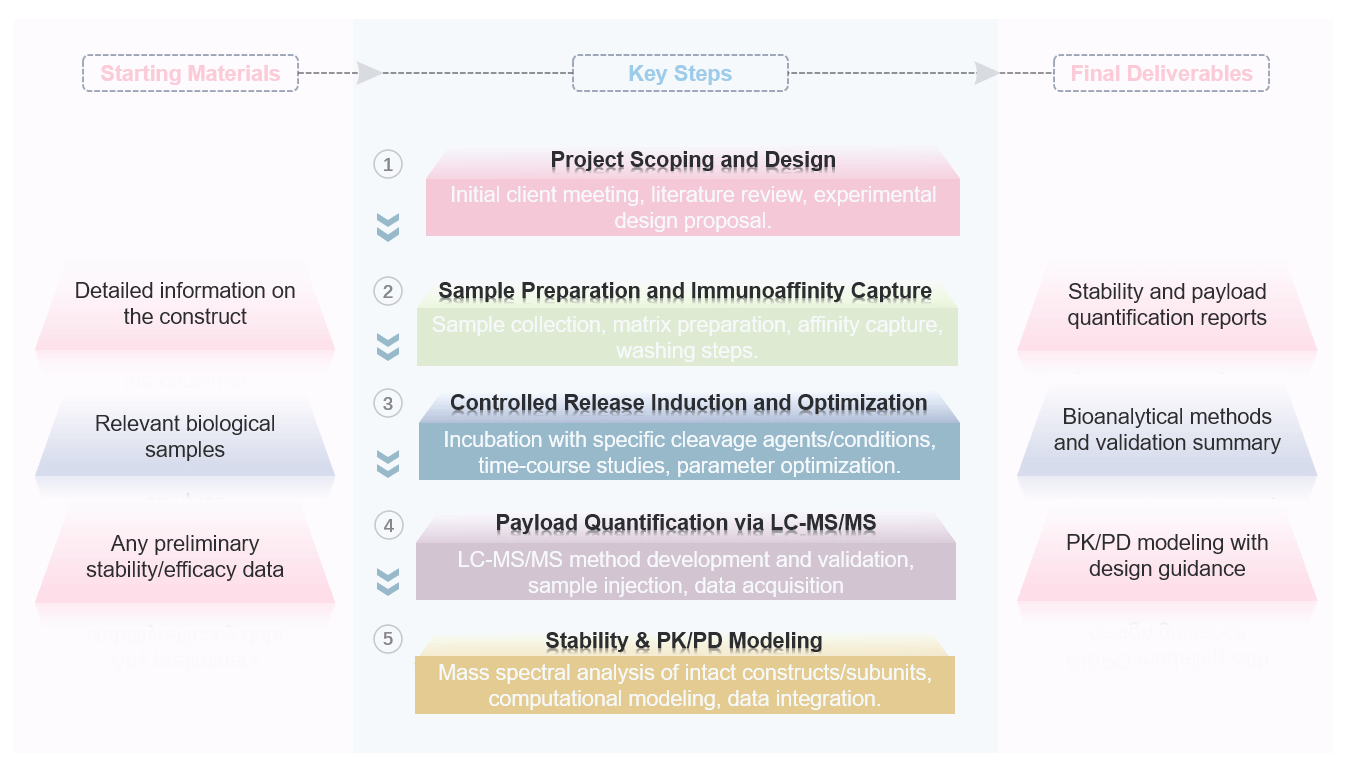
Key Advantages
- Customized Assay Development: We develop and optimize bespoke bioanalytical assays tailored to your specific responsive construct, be it a novel ADC linker, nanoparticle, or complex multi-component system.
- Comprehensive Stability Profiling: We offer in-depth analysis of your construct's stability across various biological matrices and physiological conditions, ensuring systemic integrity.
- Precise Triggered Release Characterization: Our service rigorously quantifies payload release kinetics at the target site, mimicking specific biological triggers.
- Mechanism-Based Toxicity Deconvolution: Through sophisticated PK/PD modeling, we identify specific analytes responsible for toxicities, enabling targeted design modifications for enhanced safety.
- Rational Design Guidance: Our seasoned experts translate complex data into actionable recommendations for optimizing your construct's design.
- Advanced In Vitro Predictive Models: We utilize cutting-edge 3D cell culture models, like tumor spheroids, to provide physiologically relevant data on drug penetration and efficacy, significantly improving preclinical predictability.
- Integrated Analytical Platforms: Benefit from our comprehensive suite of integrated analytical technologies, including high-resolution LC-MS/MS, orthogonal stability assays, and advanced computational modeling, all at Creative Biolabs.
Representative Data
Summary: This study developed a dual-responsive peptide-drug conjugate nanoparticles, combining lytic peptide and paclitaxel via legumain-cleavable and pH-sensitive linkers. The nanoparticles showed good stability, prolonged blood circulation, and pH/legumain-triggered sequential release of paclitaxel and lytic peptide. In vitro, they exhibited cytotoxicity against MCF-7, HCT116, and 4T1 cells, with the lytic peptide disrupting cell membranes. In vivo, the nanoparticles enhanced antitumor efficacy in 4T1 tumor-bearing mice, with lower toxicity compared to taxol. Conclusions: The nanoparticles enable targeted, controlled co-delivery of paclitaxel and the lytic peptide, achieving synergistic cancer therapy, offering a strategy for precise drug delivery.
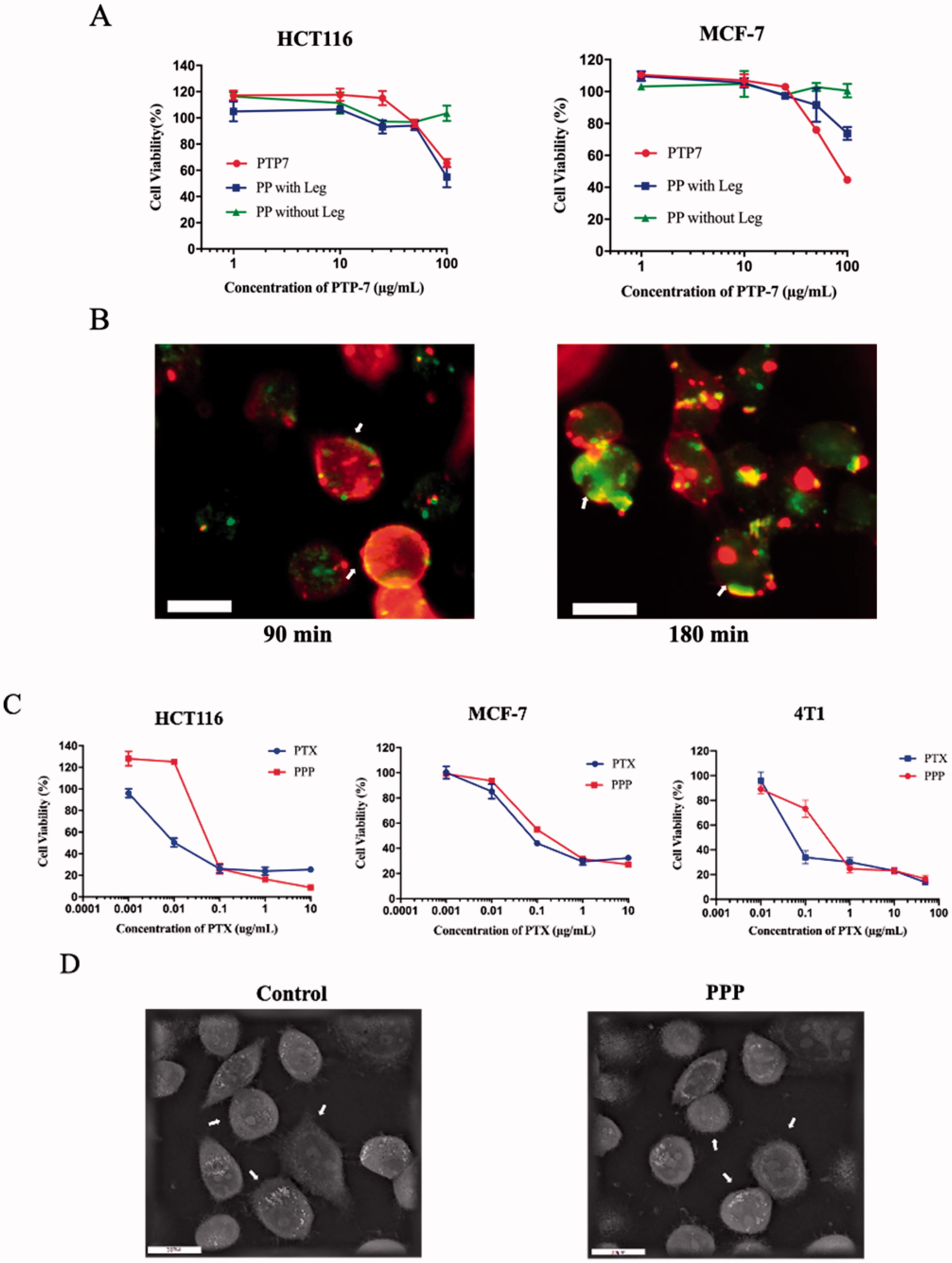 Fig.1 The process of cellular drug uptake.1
Fig.1 The process of cellular drug uptake.1
FAQs
How does Creative Biolabs ensure the accuracy of drug release measurements?
We employ highly sensitive LC-MS/MS for precise payload quantification, complemented by orthogonal methods like antibody-subunit analysis. Our rigorous optimization protocols for release conditions (e.g., pH, temperature, reducing agents) ensure that the measured release kinetics accurately reflect the construct's behavior.
Can your service help optimize my construct's design to reduce toxicity?
Absolutely. Our mechanism-based PK/PD modeling capabilities allow us to identify the specific analytes responsible for observed toxicities. By understanding these drivers, we can simulate and suggest modifications to your construct's design (e.g., linker stability, conjugation site) to mitigate off-target effects and improve its therapeutic index.
What types of responsive constructs can Creative Biolabs assess?
We specialize in assessing a wide range of responsive constructs, including ADCs with various linker chemistries (e.g., disulfide-linked, protease-cleavable) and stimuli-responsive nanoparticles (e.g., thermoresponsive, pH-responsive). If you have a unique construct, please reach out to discuss its specific assessment needs.
Why Choose Us?
At Creative Biolabs, we bring this comprehensive expertise to the forefront. Our deep understanding of ADC linker chemistry, conjugation site effects, and advanced PK/PD modeling, combined with our proficiency in designing and characterizing smart nanoparticles, positions us as an invaluable partner.
Customer Reviews
"Using Creative Biolabs' responsive construct stability assessment service in our ADC research has significantly improved our understanding of linker stability and payload release kinetics. Their detailed LC-MS/MS data and orthogonal analyses provided the clarity we needed to optimize our lead candidate." - 2024, Dr. L***a M.
"We relied on Creative Biolabs' responsive construct stability assessment service for the comprehensive biocompatibility testing of our novel nanoparticles. Their thorough evaluation, including protein adsorption, hemolysis, and thrombogenicity, gave us immense confidence in our formulation's systemic safety, far exceeding our previous internal efforts." - 2024, Dr. K***e R.
How to Contact Us
Don't hesitate to get in touch with our passionate team - we're ready to dive into the details, answer all your questions, and have a thoughtful conversation about your project's unique goals, challenges, and aspirations. Whether you need more information, want to brainstorm ideas, or simply discuss next steps, we're here to support you every step of the way.
Reference
- Zheng, Shanshan et al. "Legumain/pH dual-responsive lytic peptide-paclitaxel conjugate for synergistic cancer therapy." Drug delivery vol. 29,1 (2022): 1764-1775. Distributed under Open Access License CC BY 4.0, without modification. DOI: https://doi.org/10.1080/10717544.2022.2081380
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

