Internalizing Antibody Production and QC Service
Internalizing Antibodies: A Targeted Therapeutic Approach
Internalizing antibodies are a class of therapeutic agents that trigger cellular uptake via endocytosis upon binding to their target antigen on a cell surface. This process enables the targeted delivery of therapeutic payloads, such as immunotoxins and antibody-drug conjugate, directly into cells. By delivering therapeutic agents directly to target cells, internalizing antibodies can enhance efficacy, minimize off-target effects, and improve overall treatment outcomes. This targeted approach holds significant promise for treating a wide range of diseases and cancers.
The Advantages of Internalizing Antibody for Antibody-based Therapeutics
Internalizing antibody offers several significant advantages for antibody-based therapeutics, including:
- Targeted drug delivery
- Enhanced therapeutic efficacy
- Reduced systemic toxicity
- Modulation of cellular signaling
- Improved imaging and diagnostics
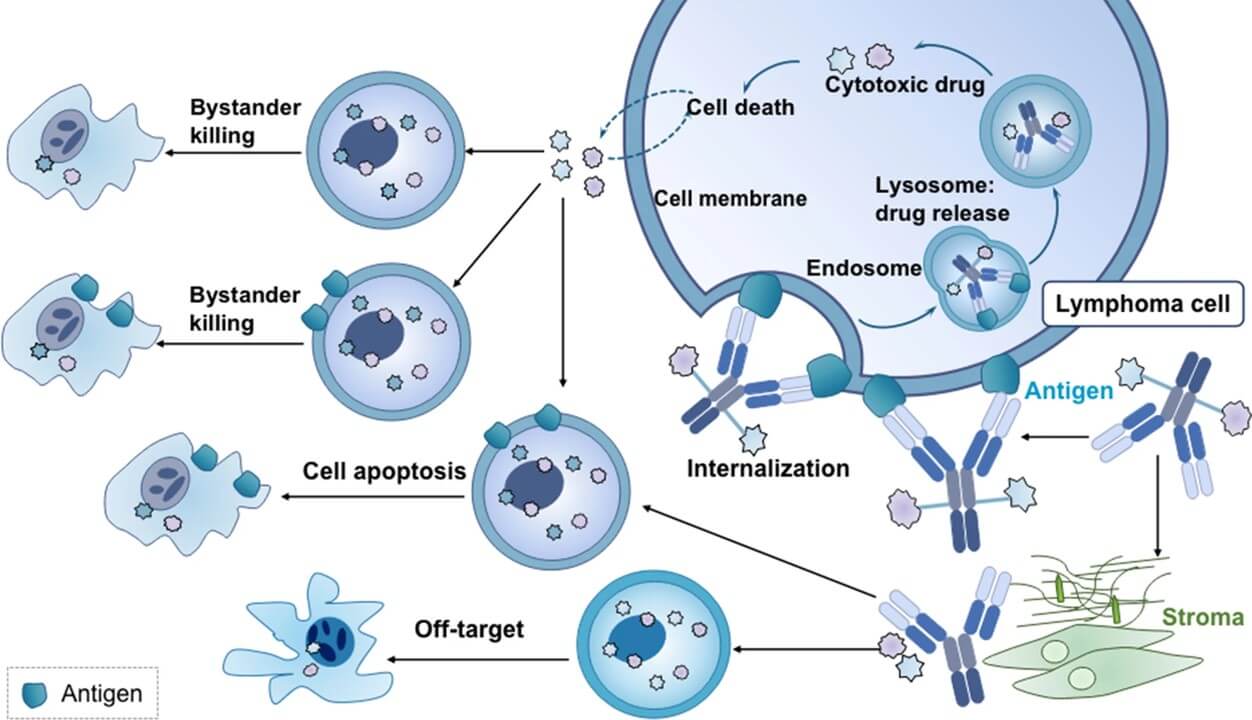 Fig.1 The mechanism of action of antibody-drug conjunction (ADCs).1
Fig.1 The mechanism of action of antibody-drug conjunction (ADCs).1
Internalizing Antibody Production and QC Service at Creative Biolabs
Creative Biolabs provides a comprehensive internalizing antibody production and QC service to help develop antibody-based therapeutics. Our end-to-end services involve multiple steps from antibody sequence optimization, gene synthesis, and vector construction to antibody expression, purification, and characterization. We provide a variety of eukaryotic expression systems to ensure the correct folding and modification of the target antibody, as well as a variety of advanced purification technologies to achieve high purity and high yield of the target antibody. In addition, we employ a variety of advanced technologies to characterize the target antibody, such as flow cytometry, WB, ELISA, etc.
In particular, we introduce real-time live cell imaging technology to characterize the internalization efficiency of the internalizing antibody. Moreover, we also supply a series of pH-sensitive dyes to adapt to pH changes during antibody internalization detection to ensure accurate and rapid characterization. Leveraging our robust antibody production platform, we have great confidence in delivering outstanding results in customers' desired turnaround.
 Fig.2 Our service process.
Fig.2 Our service process.
Selective Expression Systems
A suitable expression system is crucial for the quality, yield, and functional modification of the expressed antibodies. To guarantee optimal synthesis of these therapeutic compounds, we have validated several expression systems, including mammalian cell lines as well as insect cell lines to ensure optimal production of these therapeutic agents.
- CHO cells
- HEK293 cells
- etc.
Internalizing Antibody Characterization
We conduct comprehensive characterization to ensure the quality and consistency of internalizing antibodies, which includes:
- Structural Characterization: Primary structure, secondary and tertiary structure, post-translational modifications;
- Physicochemical Characterization: Molecular weight, isoelectric point (pI), thermal stability;
- Functional Characterization: Binding affinity, specificity, internalization efficiency, biological activity.
Highlight Features
- Capabilities to meet diverse project needs, from a modest to huge scale on manufacturing.
- Compliance with industry regulatory specifications for quality assurance.
- Rigorous purification to ensure optimal performance.
- Economical solutions.
- Efficient production processes for timely delivery.
- High-quality, high-yield antibody production.
Creative Biolabs, a pioneer in antibody invention, is committed to improving the development and production of excellent antibodies. We are constantly optimizing our techniques to ensure an efficient and reliable supply of high-grade antibodies. If you are seeking a dependable collaborator for your internalizing antibody projects, please contact us immediately.
- Chu, Yurou, Xiangxiang Zhou, and Xin Wang. "Antibody-drug conjugates for the treatment of lymphoma: clinical advances and latest progress." Journal of Hematology & Oncology 14.1 (2021): 88. Distributed under Open Access License CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

