

Ifabotuzumab Overview
Introduction of Ifabotuzumab
Ifabotuzumab (formerly KB004) is a first-in-class therapeutic non-fucosylated IgG1 (human f-allotype) monoclonal antibody being developed by Humanigen (formerly KaloBios Pharmaceuticals) for the treatment of cancer. The drug was licensed from the Ludwig Institute for Cancer Research. The molecule contains a human antibody constant region with V-regions that are 92% identical to human germ-line sequences. Ifabotuzumab targets the ephrin type-A receptor 3 (EphA3) tyrosine kinase. In non-clinical studies, Ifabotuzumab demonstrated potent ex-vivo cell killing of EphA3+ tumor cells obtained from patients with hematologic malignancies.
Mechanism of Action of Ifabotuzumab
Ephrin receptors and their ligands represent a class of cell-cell communication molecules with developmental functions. The Ephrin receptor family is divided into 2 groups, EphA and EphB, based on the sequence homology of their extracellular domains and binding affinity to their corresponding ligands, either glycosyl-phosphatidyl-inositol–linked ligands (ephrin-A ligands) or transmembrane ligands (ephrin-B ligands). Both receptor and ligand are membrane bound. Direct receptor-ligand contact causes receptor clustering, and activation of signaling cascades both in the receptor bearing and ligand bearing cells. This unique mechanism of bi-directional signaling is thought to be critical for the cell motility functions of these proteins. EphA3 was first isolated as an antigen on the surface of a human pre-B-cell acute lymphoblastic leukemia (ALL) cell line. Since then, EphA3 gene copy number changes have been associated with most hematologic malignancies. These gene copy number differences correlated with EphA3 mRNA levels. Phenotype analysis of AML patients demonstrated relatively higher expression of EphA3 in leukemic stem cells compared to their normal counterparts. Transcriptional profiling of EphA3 mRNA in patients with hematologic malignancies has demonstrated up-regulation of EphA3 gene expression in bone marrow mesenchymal cells following exposure to hypoxic conditions. These data suggest that hypoxia may be an important factor in inducing expression of EphA3 on tumor endothelial cells. In murine models, targeting EphA3 on tumor vasculature using anti-EphA3 antibodies reduced tumor microvessel density and stromal tissue, producing an anti-tumor effect. Importantly, increased bone marrow micro-vessel density has been reported in patients with acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS). The interaction between microvessel endothelial cells and AML blasts increases proliferation and inhibits apoptosis of naïve human AML blasts. In addition, AML patients with higher baseline microvessel density have shorter overall survival. In B-cell chronic lymphocytic leukemia (CLL), localized hypoxia, hypoxia inducible factor (HIF)-1 up-regulation, and dysregulated angiogenesis have been reported. Because EphA3 expression is induced by hypoxia, it seems likely that EphA3 is expressed on micro-vessels in leukemic bone marrow and could be targeted for therapeutic gain, possibly leading to disruption of the leukemic stem cell niche. Furthermore, in vitro experiments suggest that the expression of EphA3 on leukemic cells can lead to an integrin-independent adhesion to stromal cells. This interaction might also have therapeutic relevance. Clinical trials with anti-angiogenic agents in hematologic malignancies are currently underway and highlight the potential of this approach. Since EphA3 is an oncofetal antigen with restricted tissue expression, the development of a targeted agent holds promise. Four mechanisms of cell killing have been described: direct induction of apoptosis, activation of antibody-dependent cell-mediated cytotoxicity (ADCC), disruption of tumor vasculature and inhibition of survival signaling from the marrow microenvironment.
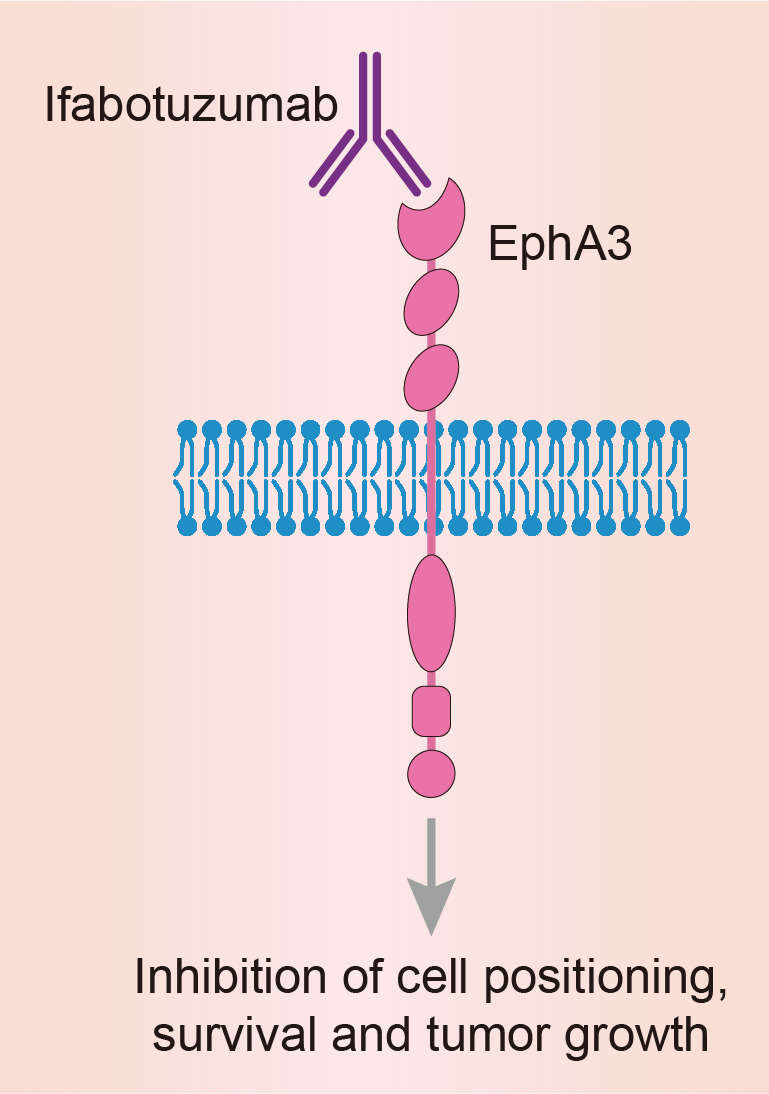 Fig.1 Mechanism of Action of Ifabotuzumab
Fig.1 Mechanism of Action of Ifabotuzumab
Table 1. Clinical Projects of Ifabotuzumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03374943 | Recruiting | Glioblastoma | Olivia Newton-John Cancer Research Institute | December 15, 2017 |
What We Provide
Therapeutic Antibody
Ifabotuzumab
We provide high-quality Ifabotuzumab (IgG1κ type) for immunoassay techniques such as: Enzyme-Linked Immunosorbent Assay; Immunohistochemistry; Immunofluorescence; Immunoprecipitation; Flow Cytometry; Functional Studies. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
References
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=KB004
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

