Elgemtumab Overview
Introduction of Elgemtumab
Elgemtumab, also known as LJM716, is a high- affinity IgG1κ type monoclonal antibodies selected from a Human Combinatorial Antibody Library (HuCAL). It can specifically bind to ERBB3 to neutralize multiple modes of ERBB3 activation. ERBB3 is the abbreviation of receptor tyrosine-protein kinase erbB-3, also known as HER3 (human epidermal growth factor receptor 3). It is a member of the ERBB family of receptor tyrosine kinases (RTK). ERBB3 mediated activation of PI3K signaling when inappropriate ERBB2/ERBB3 dimerization, which usually happens in HER2 or neuregulin (NRG) over-expression cancer cells. Thus, ERBB3 is consideration as a mediator of oncogenic transformation and a therapeutic target of cancer. Elgemtumab can potently inhibit the phosphorylation of ERBB3/ AKT and the proliferation of tumor cells over-expressing HER2 and NRG. Research has shown that elgemtumab can significantly inhibit tumor growth in a variety of xenograft models and induced tumor regression. In addition, elgemtumab shows synergistic function combination with trastuzumab, cetuximab or PI3K- targeted agents in a panel of in vitro cell lines. And the combination of elgemtumab with trastuzumab was efficacious in BT474, and the combination with erlotinib was efficacious in (pancreatic) tumor xenografts. Elgemtumab was developed by Memorial Sloan-Kettering Cancer Center and Novartis Oncology for the treatment of breast cancer, gastric cancer, head and neck cancer, oesophageal cancer, and solid tumours. Elgemtumab had been investigated in platinum-pretreated recurrent/metastatic patients with HNSCC in combination with cetuximab. However, the study is withdrawn. The Phase I study elgemtumab combined with trastuzumab in patients with HER2 overexpressing metastatic breast or gastric cancer is completed. The study of safety and efficacy of the combination of elgemtumab and BYL719 in patients with previously treated esophageal squamous cell carcinoma (ESCC) is also completed. Furthermore, a phase I study of elgemtumab in japanese patients with advanced solid tumors also completed. The evaluating the safety and tolerability study of elgemtumab in combination with trastuzumab, BYL719 in metastatic HER2+ breast cancer patients is ongoing. Based on the preclinical data, combining elgemtumab with other drugs such as trastuzumab, or cetuximab, may lead to greater clinical efficacy.
Mechanism of Action of Elgemtumab
The mechanism of elgemtumab inhibits multiple modes of ERBB3 activation was considered as binding to ERBB3, trapping it in an inactive conformation. The crystal structure study of elgemtumab has found that elgemtumab binds to a novel conformational epitope contained within domains 2 and 4 and appears to trap ERBB3 in the inactive conformation. Elgemtumab do not prevent ligand binding of NRG and ERBB3 nor does it affect the binding affinity of the ERBB3/NRG interaction. But it prevents the structural rearrangements required for ERBB3 activation induced by either ERBB2 or NRG. The novel mechanism of action of elgemtumab makes it becomes the first HER3 antibody to display efficacy in both HER2 and ligand driven xenograft models. In a word, elgemtumab binds to ERBB3 extracellular domain (ECD) and blocks it in tethered conformation. The inactivated form of ERBB3 blocks the PI3K/Akt signaling pathway, thereby inhibiting cellular proliferation in HER2 or NRG expressing tumor cells.
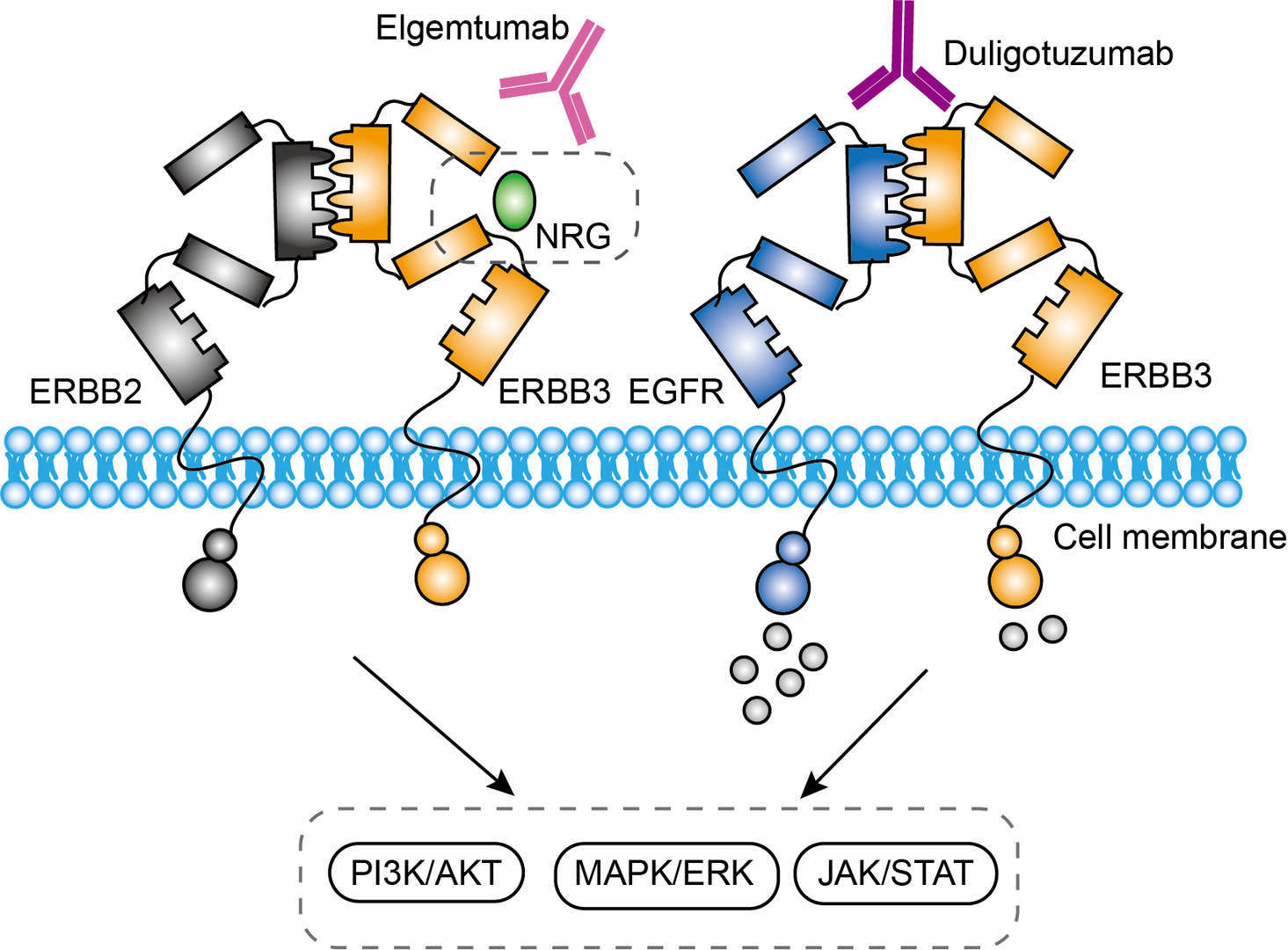 Fig.1 Mechanism of Action of Elgemtumab
Fig.1 Mechanism of Action of Elgemtumab
Table 1. Clinical Projects of Elgemtumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT02167854 | Active, not recruiting | Breast Cancer | Memorial Sloan Kettering Cancer Center | September 17, 2019 |
References
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=LJM716
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



