Polatuzumab Vedotin Overview
Introduction of Polatuzumab vedotin
Polatuzumab vedotin is an active ingredient of Polivy, a drug product for the treatment of previously treated adult patients with diffuse large B-cell lymphoma (DLBCL) in combination with bendamustine and rituximab. Polatuzumab vedotin is an antibody-drug conjugate composed of a humanized monoclonal antibody (mAb) targeting B-cell antigen receptor complex-associated protein beta chain (CD79b) and a microtubule-disrupting toxin, monomethyl auristatin E (MMAE). This drug was developed by Genentech/Roche using a proprietary technology developed by Seattle Genetics. In 2018, orphan designation was granted for polatuzumab vedotin for the treatment of diffuse large B-cell lymphoma by the European Commission to Roche Registration Limited. Based on the effective therapeutic effect of polatuzumab vedotin on DLBCL, the U.S. Food and Drug Administration (FDA) granted accelerated approval to polatuzumab vedotin, in combination with bendamustine plus rituximab on 10 June 2019. Subsequently, the European Medicines Health and Therapeutic Goods Administration of Australian Drug Regulatory Administration also approved Polivy's sales authorization from Genentech. Besides DLBCL, polatuzumab vedotin also has been investigated in the treatment of non-hodgkins lymphoma, chronic lymphocytic leukemia, follicular lymphoma. Some of the trials were complicated, and there are six clinical trials still undergoing now. For example, there is a phase Ib/II study investigating the safety, tolerability, pharmacokinetics, and efficacy of mosunetuzumab (BTCT4465A) in combination with chop or chp-polatuzumab vedotin in participants with b-cell non-hodgkin lymphoma. Furthermore, a study to evaluate the safety and efficacy of polatuzumab vedotin in combination with rituximab, gemcitabine and oxaliplatin compared to rituximab, gemcitabine and oxaliplatin alone in participants with relapsed or refractory diffuse large B-cell lymphoma is recruiting. The recent events of polatuzumab vedotin is that Chugai Pharmaceutical, another developer, adverse events data from a phase ii (jo40762/p-drive) trial diffuse large B-cell lymphoma and announces intention to submit NDA to Ministry of Health, Labour and Welfare for diffuse large B-cell lymphoma in Japan on February 2020. At the same period, a phase-II clinical trials in diffuse large B cell lymphoma is undergoing in United Kingdom (IV).
Mechanism of Action of Polatuzumab vedotin
The therapeutic target molecule CD79b is a transmembrane protein expressed on the surface of B-cells as part of the B-cell antigen receptor complex. CD79b plays a critical in expression and function of the B-cell antigen receptor together by forming a disulfide-linked heterodimeric complex with Ig-alpha. In addition, CD79b is expressed on the surface of almost all types of malignant B-cells. Thus, targeting CD79b therapy for B-cell diseases has become a new strategy. Polatuzumab vedotin is a first in class antibody-drug conjugate targeting CD79b in combination with bendamustine plus rituximab for the treatment of DLBCL. Studies have shown that polatuzumab vedotin effectively and selectively kill cells expressing CD79b in some in vitro models. Polatuzumab vedotin contains anti-CD79b monoclonal antibody produced by mammalian cells and a chemical synthesis molecule MMAE, and a cleave-able citrulline-valine (VC) linker. The drug-to-antibody ration of polatuzumab vedotin is 3.5. The mechanism of polatuzumab vedotin is just like other ADC. Upon administration, polatuzumab vedotin selectively binds to B-cell surface antigen CD79b, promoting the internalization. Once inside the cell, proteolytic cleavage makes the MMAE release and then bind to microtubules, leading to a G2/M phase arrest and tumor cell apoptosis.
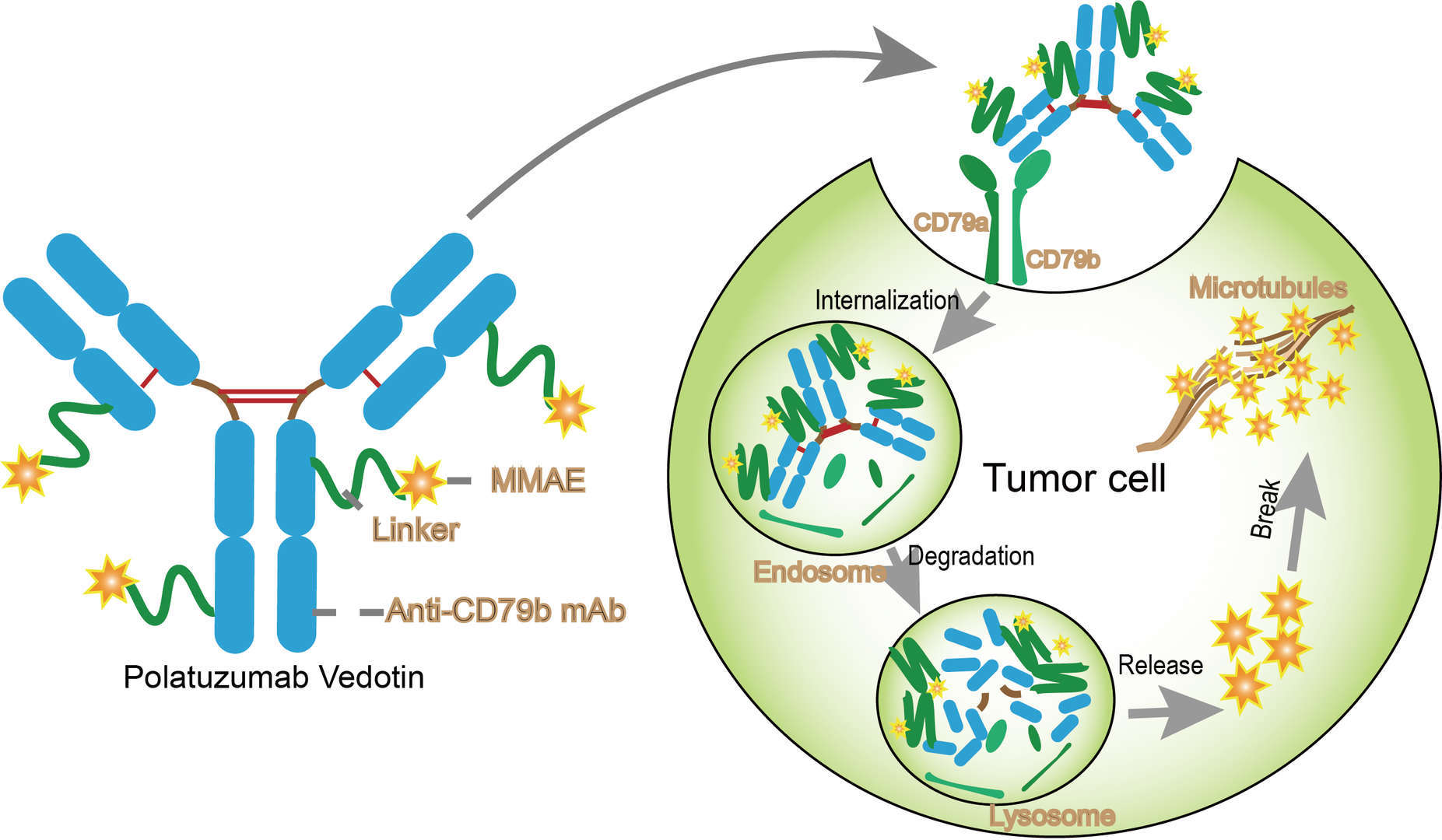 Fig.1 Mechanism of Action of Polatuzumab vedotin
Fig.1 Mechanism of Action of Polatuzumab vedotin
Table 1. Clinical Projects of Polatuzumab vedotin*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT04231877 | Not yet recruiting | Aggressive Non-Hodgkin Lymphoma; ALK-Positive Large B-Cell Lymphoma; B-Cell Lymphoma, Unclassifiable, With Features Intermediate Between Diffuse Large B-Cell Lymphoma and Classic Hodgkin Lymphoma; EBV-Positive Diffuse Large B-Cell Lymphoma, Not Otherwise Specified; High Grade B-Cell Lymphoma With MYC and BCL2 and/or BCL6 Rearrangements; High Grade B-Cell Lymphoma, Not Otherwise Specified; High Grade B-Cell Lymphoma, Not Otherwise Specified;Primary Mediastinal (Thymic) Large B-Cell Lymphoma; T-Cell/Histiocyte-Rich Large B-Cell Lymphoma; | University of Washington | February 18, 2020 |
| NCT03274492 | Recruiting | Diffuse Large B-Cell Lymphoma | Hoffmann-La Roche | February 12, 2020 |
| NCT03671018 | Recruiting | B-cell Non-Hodgkin Lymphoma | Hoffmann-La Roche | February 25, 2020 |
| NCT04236141 | Not yet recruiting | Diffuse, Large B-Cell, Lymphoma | Hoffmann-La Roche | February 17, 2020 |
| NCT04182204 | Recruiting | Diffuse Large B-Cell Lymphoma | Hoffmann-La Roche | February 19, 2020 |
| NCT02611323 | Recruiting | Non-Hodgkin's Lymphoma | Hoffmann-La Roche | February 5, 2020 |
| NCT02600897 | Recruiting | Relapsed or Refractory Follicular Lymphoma, Relapsed or Refractory Diffuse Large B-Cell Lymphoma | Hoffmann-La Roche | February 17, 2020 |
| NCT03533283 | Recruiting | Non-Hodgkins Lymphoma | Hoffmann-La Roche | February 11, 2020 |
| NCT02257567 | Active, not recruiting | Lymphoma | Hoffmann-La Roche | January 21, 2020 |
| NCT03677141 | Recruiting | B-cell Non-Hodgkin Lymphoma | Hoffmann-La Roche | February 5, 2020 |
| NCT02729896 | Active, not recruiting | Lymphoma | Hoffmann-La Roche | January 3, 2020 |
Table 2. Approved Drugs of Polatuzumab vedotin**
| INN (trade name) | Therapeutic area | Dose | Strength | Route | Company | Marketing start | Market |
| Polivy | Relapsed or refractory diffuse large B-cell lymphoma | Powder for injection | 140 mg/vial | Injection | Genentech, Inc | June 10, 2019 |
|
| Polivy | Relapsed or refractory diffuse large B-cell lymphoma | Powder for injection | 140 mg/vial | Infusion | Roche Registration GmbH | January 16,2020 |
|
| Polivy | Relapsed or refractory diffuse large B-cell lymphoma | Powder for injection | 140 mg/vial | Intravenous infusion | Roche Products Pty Ltd | October 18, 2019 |
|
References
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Polatuzumab+vedotin
** Information presented in the table were collected from the following website:
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=%20761121
https://www.ema.europa.eu/en/medicines/human/EPAR/polivy
http://search.tga.gov.au/s/search.html?collection=tga-artg&profile=record&meta_i=314866
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

