Tezepelumab Overview
Introduction of Tezepelumab
Tezepelumab (formerly MEDI9929 and AMG 157) is a human monoclonal antibody (mAb) developed in collaboration by MedImmune (now part of AstraZeneca) and Amgen. It blocks thymic stromal lymphopoietin (TSLP) and was designed to for the treatment of asthma and atopic dermatitis (AD). It is in Phase III trials as of August 2019.
Mechanism of Action of Tezepelumab
TSLP is a pleiotropic cytokine originally characterized as a lymphocyte growth factor. TSLP, IL-33, and IL-25 are alarmins overexpressed in airway epithelial cells which have been associated with the pathogenesis of asthma. Human TSLP exerts its biological activities by binding to a high-affinity heteromeric receptor complex composed of TSLPR and IL-7 receptor-α (IL-7Rα). Human TSLP, positively charged, binds to TSLPR, negatively charged, with high affinity; then, IL-7Rα binds to the TSLP:TSLPR binary complex. The ternary complex, TSLPR:TSLP:IL-7Rα, initiates signaling in cells co-expressing TSLPR and IL7Rα. TSLP is expressed predominantly by lung and gut epithelial cells, keratinocytes and dendritic cells (DCs). TSLP can be produced also by airway smooth muscle (ASM) cells, human DCs and mast cells, human monocytes, macrophages and granulocytes, and murine basophils. At the barrier surfaces (e.g., airway epithelium) TSLP is an upstream cytokine that activates TSLPR+ DCs to induce functional T helper type 2 (Th2) cells and T follicular helper (Tfh) cells. TSLP also possesses a plethora of pleiotropic properties mediated by the activation of a broad range of immune and non-immune cells. A wide spectrum of stimuli involved in the pathogenesis of different phenotypes/endotypes of asthma such as allergens, cytokines (e.g., TNF-α, IL-1β), respiratory viruses, bacterial and fungal products, cigarette smoke extracts and tryptase induce the expression of TSLP from different target cells.
TSLP-activated DCs prime CD4+ T cells to polarize toward Th2 cells (e.g., production of IL4, IL-5, and IL-13). TSLP produced by activated lung epithelial cells can cause goblet cell hyperplasia and mucus production. TSLP targets group 2 innate lymphoid cells (ILC2s) and drives the development of Th2 cells. TSLP also provides critical signals for human B cell proliferation and T follicular helper cells (Tfh) differentiation. Human monocytes do not express TSLPR and IL-7Rα but their stimulation with lipopolysaccharide (LPS) induces the expression of TSLPR complex on a percentage of monocytes. TSLP activates human eosinophils through the engagement of TSLPR and IL-7Rα. TSLP promoted peripheral basophilia and that TSLPR-expressing basophils play a role in Th2-dependent immunity in mice. Collectively, these findings demonstrate that TSLP might orchestrate a plethora of immune cells involved in the pathogenesis of different phenotypes/endotypes of asthma.
By binding to TSLP, tezepelumab prevents its interaction with the TSLP receptor complex and inhibits multiple downstream inflammatory pathways. Preclinical data support the role of TSLP in both asthma and AD, indicating that tezepelumab may be effective as a treatment in both diseases.
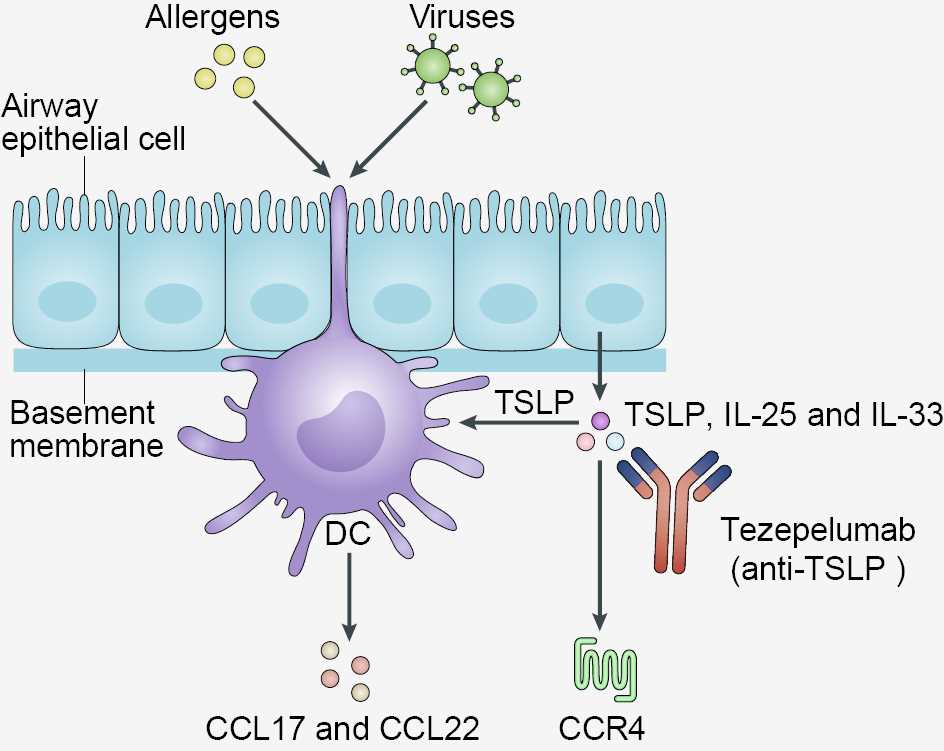 Fig.1 Mechanism of Action of Tezepelumab
Fig.1 Mechanism of Action of Tezepelumab
Table 1. Clinical Projects of Tezepelumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT04362410 | Recruiting | Healthy Subjects | AstraZeneca | April 24, 2020 |
| NCT03968978 | Active, not recruiting | Asthma | AstraZeneca | May 30, 2019 |
| NCT04039113 | Active, not recruiting | Chronic Obstructive Pulmonary Disease (COPD) | AstraZeneca | July 31, 2019 |
| NCT03809663 | Recruiting | Atopic Dermatitis | Amgen | January 18, 2019 |
| NCT03927157 | Active, not recruiting | Asthma | AstraZeneca | April 25, 2019 |
| NCT04048343 | Active, not recruiting | Severe Asthma | AstraZeneca | August 7, 2019 |
| NCT03347279 | Active, not recruiting | Asthma | AstraZeneca | November 20, 2017 |
| NCT03688074 | Active, not recruiting | Asthma; Bronchial Diseases; Respiratory Tract Diseases; Lung Diseases, Obstructive; Lung Diseases; Respiratory Hypersensitivity; Hypersensitivity, Immediate; Hypersensitivity; Immune System Diseases; | AstraZeneca | September 28, 2018 |
| NCT03706079 | Enrolling by invitation | Asthma | AstraZeneca | October 15, 2018 |
| NCT03406078 | Active, not recruiting | Asthma | AstraZeneca | January 23, 2018 |
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

