Andecaliximab Overview
Introduction of Andecaliximab
Andecaliximab, a recombinant chimeric IgG4 monoclonal antibody (mAb), was engineered to remove T-cell epitopes to reduce immunogenicity risk. Andecaliximab selectively binds and inhibits matrix metalloproteinase-9 (MMP9) with minimal cross-reactivity to other matrix metalloproteinases, including the highly homologous matrix metalloproteinase-2 (MMP-2). Andecaliximab is under development for the treatment of cystic fibrosis, gastric cancer, pancreatic cancer, non-small cell lung cancer (NSCLC), rheumatoid arthritis (RA), Crohn's disease (CD), and ulcerative colitis (UC). In a recent phase 1 dose-escalation study in patients with UC, andecaliximab had good tolerability and was associated with a numerically greater percentage of clinical, endoscopic, and histological responses in patients relative to placebo over a 5-week treatment period. A phase 2/3 trial, evaluating the safety and efficacy of andecaliximab to induce and maintain clinical remission in patients with moderate to severe UC, was initiated. A planned interim futility analysis following an 8-week induction period in the first 150 patients resulted in the termination of the study due to lack of efficacy.
Mechanism of Action of Andecaliximab
Matrix metalloproteinase (MMP) family are involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, angiogenesis, bone development, wound healing, cell migration, learning and memory, as well as in pathological processes, such as arthritis, intracerebral hemorrhage, and metastasis. Most MMPs are secreted as inactive proproteins which are activated when cleaved by extracellular proteinases. The enzyme encoded by this gene degrades type IV and V collagens and other extracellular matrix proteins. Studies in rhesus monkeys suggest that the enzyme is involved in IL-8-induced mobilization of hematopoietic progenitor cells from bone marrow, and murine studies suggest a role in tumor-associated tissue remodeling. Specifically, the MMP9 exhibits increased mucosal protein and mRNA expression, serum antigen concentrations, and activity in patients with UC compared with healthy controls. Furthermore, relative to healthy controls and patients with other types of IBD, faecal MMP9 concentrations are significantly increased in patients with UC and correlate with clinical and endoscopic activity scores. Targeted deletion or pharmacological inhibition of MMP9 attenuates colonic damage in experimental colitis animal models, indicating that MMP9-specific inhibition may represent a viable approach to the treatment of UC. Andecaliximab binds MMP9 at the junction between the propeptide and catalytic domains, distal to the active site, and acts by preventing the activation of inactive zymogen. In addition, this antibody also acts as an allosteric inhibitor.
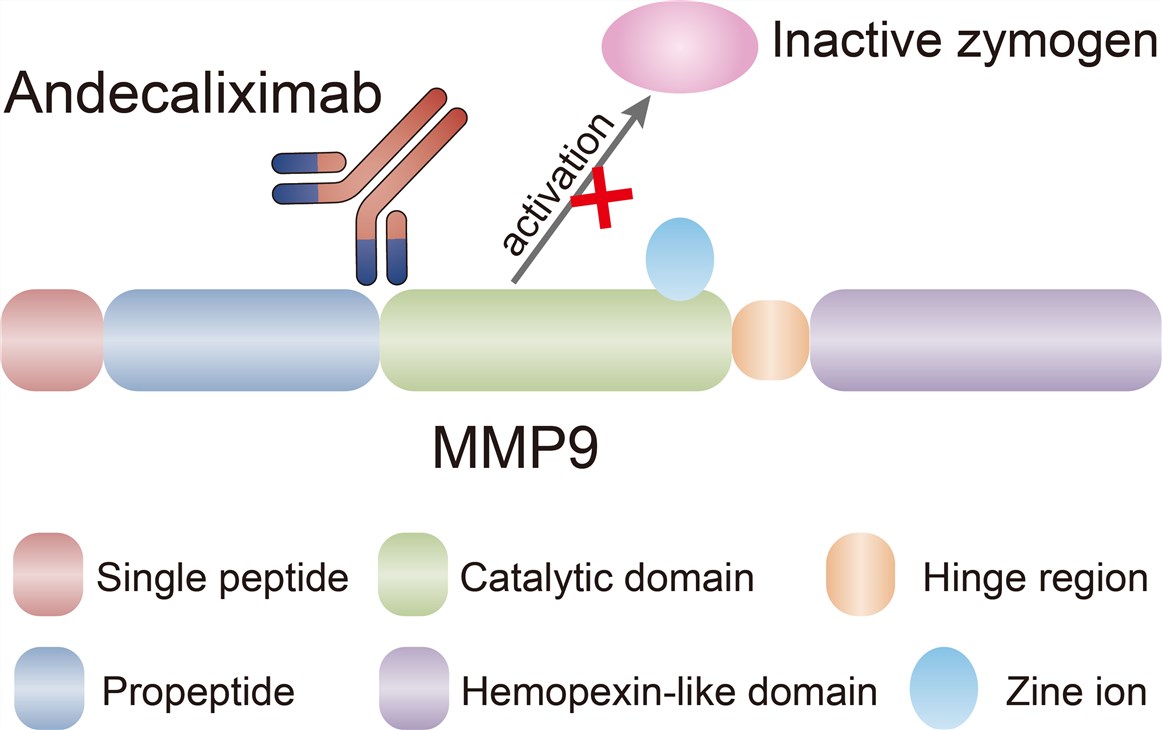
Fig.1 Mechanism of Action of Andecaliximab
Table 1. Clinical Projects of Andecaliximab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT02862535 | Active, not recruiting | Gastric Adenocarcinoma, Gastroesophageal Junction Adenocarcinoma, Gastric Adenocarcinoma | Gilead Sciences | August 11, 2016 |
| NCT02545504 | Active, not recruiting | Gastric Adenocarcinoma | Gilead Sciences | September 10, 2015 |
| NCT02864381 | Active, not recruiting | Gastric Adenocarcinoma, Gastroesophageal Junction Adenocarcinoma | Gilead Sciences | August 12, 2016 |
| NCT01803282 | Active, not recruiting | Tumors, Pancreatic Cancer, Non-small Cell Lung Cancer, Esophagogastric Cancer, Colorectal Cancer, Breast Cancer | Gilead Sciences | March 4, 2013 |
Reference
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Andecaliximab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



