Enzyme-linked Immunosorbent Assay Protocol & Troubleshooting
Enzyme-linked immunosorbent assay (ELISA) is a plate-based method for the quantitative detection of antigens in samples. ELISA has the advantage of being convenient, rapid and simple to perform. The assay can be easily expanded to set high throughputs and a large number of samples can be processed in parallel. ELISA is based on antigen-antibody interactions and there are four main types: direct, indirect, competitive and sandwich.
Sandwich ELISA is one of the more commonly used ELISA methods. Below we provide the general protocol for our sandwich ELISA. The detection and quantification of the target protein in the assay is accomplished by using a highly specific antibody. The analyte is bound between two primary antibodies, each of which detects a different epitope of the antigen.
Solutions and Reagents
| Stages | Solutions and Reagents |
| Preparation | Phosphate buffer (PBS), capturing antibody, dilution buffer, blocking solution |
| Detection | Biotinylated detection antibody, substrate solution, stop solution, washing buffer |
Enzyme-linked Immunosorbent Assay Procedure
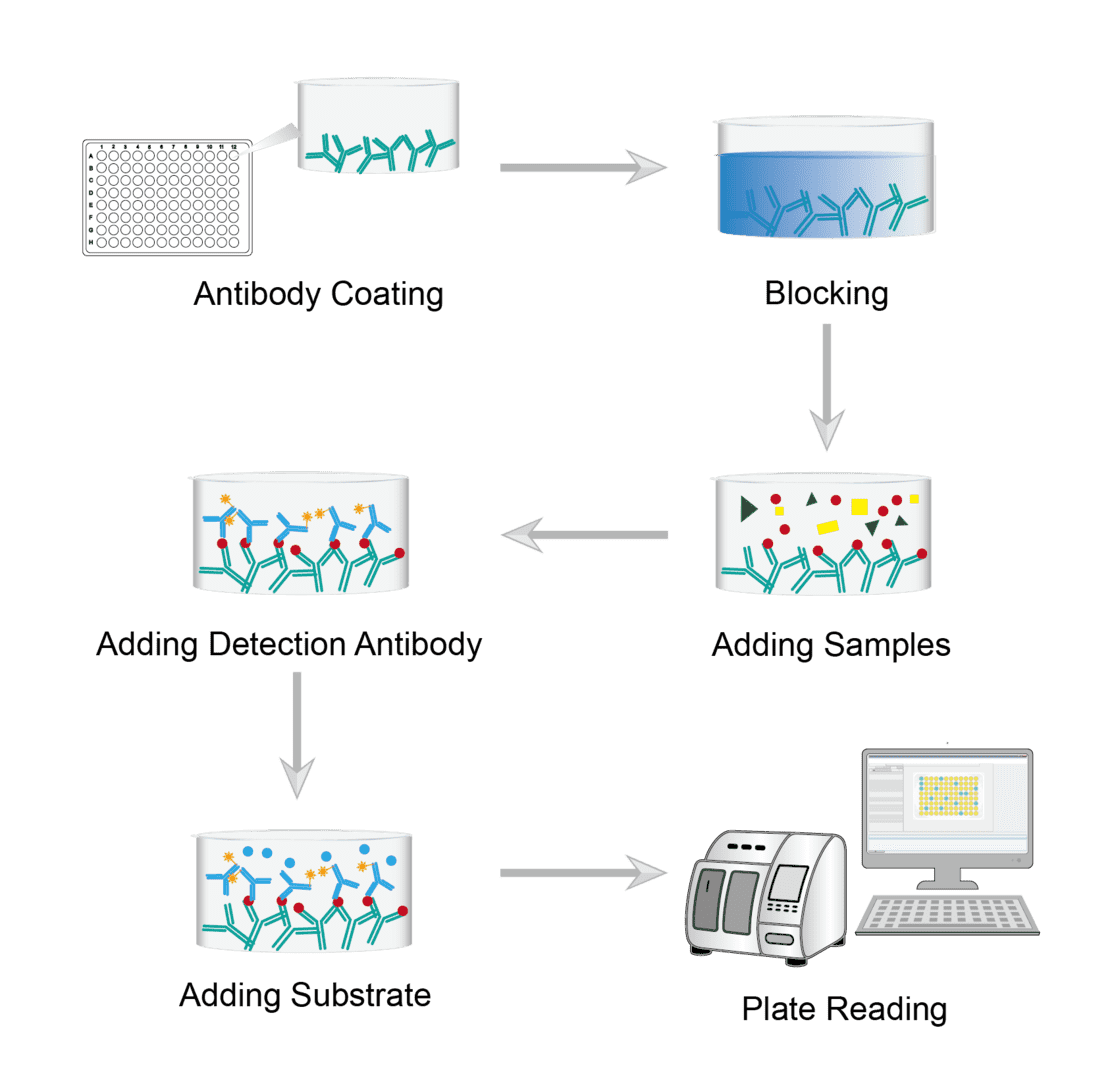
Dilute the antibody to the appropriate concentration with the encapsulation buffer. Then add the diluted capture antibody to the wells of the microtiter plate. Incubate overnight at 4°C under cover. The target protein specific capture antibody is attached to the microplate by incubation. Finally remove the coating solution and wash the plate with PBS buffer.
Add a quantitative amount of blocking buffer to each well. Cover for incubation to block residual protein binding sites of the capture antibody. Then wash each well with PBS to remove the blocking solution.
Next add the sample to the wells and incubate covered. The antigen in the sample is bound to the capture antibody. Finally wash again to wash away any unbound material.
Add a quantitative dilution of the enzyme-conjugated detection antibody to each well. Cover and incubate at room temperature. During incubation, the enzyme-conjugated detection antibody binds to a second site on the target antigen. Then wash with PBS several times and unbound antibody is washed away.
Prepare the substrate solution immediately prior to use. Add the required amount of substrate solution to each well. Allow the color to develop at room temperature. The addition of enzyme substrates allows for the indirect detection of bound proteins. The substrate is converted by the enzyme on the detection antibody, producing a color change whose intensity is proportional to the amount of antigen present.
Place the plate into the microplate reader for reading. Read the absorbance of each well. Prepare a standard curve from the serial dilution data. Interpret the concentration of the sample from this standard curve.
Troubleshooting
ELISA is a common analytical technique used in many research and biotechnology laboratories. Below is a troubleshooting guide related to common problems in ELISA assays that you can browse for reference.
No or weak signal
- Antibody causes. First consider whether the capture/detection antibody is insufficient or incorrect. You can switch to alternative antibodies and titrate the antibody reagent to determine the appropriate concentration. Next check that the capture antibody is not bound to the microtiter plate. If so, we recommend using a different coating buffer and increasing the coating time. Finally, check that the primary and secondary antibodies are compatible. Confirm that the capture and detection antibodies recognize different epitopes.
- Antigen causes. One possibility is that the protein of interest is not present. You can run a positive control or check the scientific literature to see if your sample is expected to contain protein. Another possibility is that the sample contains only a low concentration of the analyte. We recommend that you confirm sample selection or prepare a more concentrated sample.
- Reagent causes. Refer to the storage instructions of the various reagent manufacturers. Avoid unnecessary freeze-thaw cycles that can damage the quality of the reagents. When operating, pay attention to the order of reagent addition and dosage.
- Incubation time causes. It is possible that the incubation time was too short. You should incubate the sample overnight at 4°C or follow the manufacturer's guidelines. It is also possible that the incubation temperature is too low. Make sure that the incubation is carried out at the correct temperature.
- Detection method causes. The assay may not be sensitive enough. You can switch from using a direct assay to an indirect assay. Consider using a more sensitive reading for signal amplification. You remember to also check that the enzyme marker supports the selected readings.
High background
- Antigen concentration causes. Samples that are too concentrated cause high background. We recommend serial dilution of the sample to determine a more appropriate concentration.
- Antibody causes. You will need to titrate the antibody reagent in advance of the operation to determine the appropriate concentration. High antibody concentrations can lead to a high background. Interference may also be caused by an improper choice of antibody diluent. We recommend changing to a different antibody diluent. As well as non-specific binding of secondary antibodies can also cause interference. You can run a control without the primary antibody to check the specificity of the secondary antibody.
- Washing causes. Insufficient washing is also an important cause. Please increase the number and duration of washing steps. Check that the holes are completely emptied between washes. If possible, consider automated washing.
- Substrate causes. You may have prepared the colorimetric substrate too early. Prepare the colorimetric substrate immediately before use or switch to a stable substrate. Read the colorimetric assay immediately after adding the termination solution.
Poor standard curve
- Standard causes. The first common cause of poor standard curves is degradation of the standard stock solution. This can occur as a result of improper storage of reagents. You need to ensure that the solution is diluted and stored for use according to the protocol.
- Pipetting error causes. Another common cause of poor standard curve fit is pipetting error. This can result in incorrect concentrations of reagents being added to the ELISA sample wells. This is particularly the case when using multi-channel pipettes. Pipetting errors often cannot be corrected afterwards and therefore the ELISA may need to be repeated.
High coefficient of variation
- Bubble in wells causes. As you move reagents and samples into the plate, you may see small air bubbles forming in the wells. It is important to remove all these air bubbles before running the ELISA as it can affect the absorbance reading.
- Insufficient washing causes. Another potential cause of high CV is that the sample wells have not been cleaned evenly or thoroughly. The cleaning step is essential to ensure that there are no residual contaminants or non-specific binding molecules in the sample wells. Again, if you are seeing high CVs, it is recommended that the soak time in the wash step be increased by a further 30 seconds.
- Edge effect causes. Edge effects are usually caused by differences in temperature on the plate and affect the absorbance measurement of each well. To avoid edge effects, it may be necessary to use an incubator to precisely control the temperature of the plate.
Out of range
- Sample causes. Samples contain no or below detectable levels of analytes. If the sample is below the detectable level, a high sample size may be used. Alternatively, if the sample contains an analyte at a concentration above the maximum standard point, the sample may require further dilution.
- Plate sealer causes. You may not have used or reused the plate sealer. Cover the assay plate with a plate sealer during incubation. Use a fresh sealer each time the plate is opened. This will prevent wells from contaminating each other.
- Other causes. If it is not the sample, it may involve incubation, pipetting, washing, reagents, etc. and can be investigated one by one.
Products with Tested Data
At Creative Biolabs, we are dedicated to providing high-quality antibodies for various research applications. Each product in our extensive range has been rigorously tested to ensure superior reliability and efficacy. To showcase the performance of our antibodies, we have conducted numerous experiments using the ELISA method. Below, you will find a table listing a selection of our antibody products along with images from these ELISA experiments, demonstrating their proven reliability.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

