Abciximab Overview
Introduction of Abciximab
Abciximab (also known as abcixifiban or c7E3 Fab), is the Fab fragment of the chimeric human-murine, monoclonal antibody 7E3. It is composed of murine variable regions and human constant regions. Abciximab directed against the intact platelet GPIIb/IIIa (Glycoprotein IIb/IIIa) receptor and inhibits platelet aggregation. The drug was manufactured by Janssen Biologics BV and distributed by Eli Lilly under the trade name ReoPro. This Fab fragment is produced by continuous perfusion in mammalian cell culture and purified from cell culture supernatant by a series of steps including specific viral inactivation and removal procedures, digestion with papain and column chromatography. It was approval by the U.S. Food and Drug Administration early in 1994 mainly used during and after the percutaneous coronary intervention to prevent platelets form sticking together and cardiac ischemic complications in patients undergoing percutaneous coronary intervention or in patients with unstable angina not responding to conventional medical therapy when percutaneous coronary intervention is planned within 24 hours.
Mechanism of Action of Abciximab
The target receptor GPIIb/IIIa, also known as integrin αIIbβ3, is a complex formed via calcium-dependent association of gpIIb and gpIII. GPIIb/IIIa is found on the platelets surface and functions as s receptor for fibrinogen and von Willebrand factor and aids platelet activation. It was found that GPIIb/IIIa is in an inactive conformation in resting platelets, which is unable to bind to its primary ligands. During activation, the confirmation of the extracellular domain of GPIIb/IIIa changes into an active form that is much affinity for its ligands. Activated GPIIb/IIIa is essential for platelet aggregation as it is considered the final step in platelet activation leading to aggregation. Thus, GPIIb/IIIa becomes a target of several drug treatment for platelet aggregation. Abciximab is designed to binds to the intact platelets GPIIb/IIIa receptor, which alters the conformation and blocks access of large molecules to the receptor. In addition, abciximab also prevents effects by vitronectin receptor through binding to this integrin.
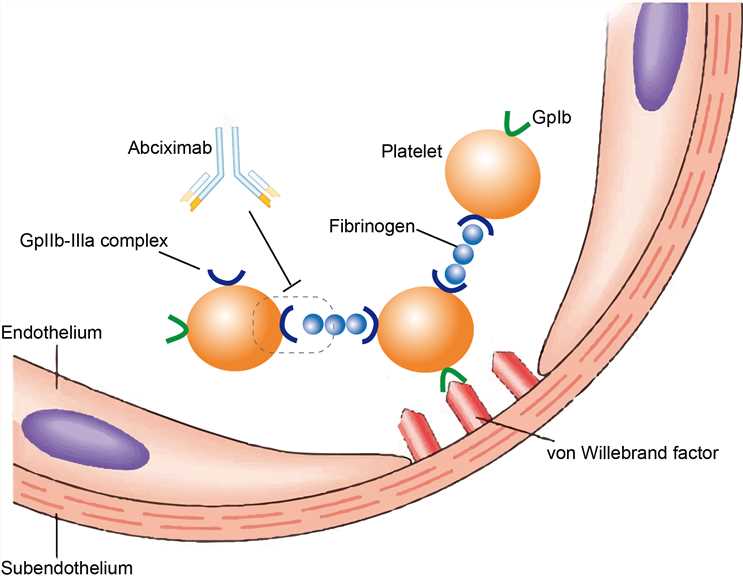 Fig.1 Mechanism of Action of Abciximab
Fig.1 Mechanism of Action of Abciximab
Table 1. Clinical Projects of Abciximab*
| NCT ID | Status | Condition | Lead Sponsor | Update Time |
| NCT03744000 | Recruiting | Acute Myocardial Infarction with ST Elevation; Anterior Wall Myocardial Infarction | Korea University Anam Hospital | November 16, 2018 |
Table 2. Approved Drugs of Abciximab**
| INN (trade name) | Therapeutic area | Dose | Strength | Route | Company | Marketing start | Market |
| REOPRO | An adjunct to percutaneous coronary intervention | Solution | 2 mg / ml | Intravenous infusion | CENTOCOR INC | December 22, 1994 |

|
| REOPRO | An adjunct to percutaneous coronary intervention | Solution | 2 mg / ml | Intravenous infusion | JANSSEN INC | October 30, 1996 |

|
| REOPRO | An adjunct to percutaneous coronary intervention | Solution | 2 mg / ml | Intravenous infusion | Janssen-Cilag Pty Ltd | October 10, 1995 |

|
Reference
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?term=Abciximab
** Information presented in the table were collected from the following website:
https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=43196
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=103575
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



