Tefibazumab Overview
Introduction of Tefibazumab
Tefibazumab is a humanized immunoglobulin G1 kappa (IgG1κ) monoclonal antibody (mAb). It was designed for the potential intravenous prevention and/or treatment of Staphylococcus aureus (S. aureus) infections. It’s a variable antigen binding region is composed of human (98%) and murine (2%) amino acid sequences. Tefibazumab specifically recognizes clumping factor A (ClfA) of S. aureus with a high affinity. ClfA, a surface adhesin protein found on virtually all strains of S. aureus, is an MSCRAMM (microbial surface components recognizing adhesive matrix molecules) protein that mediates the adhesion of S. aureus to fibrinogen. In preclinical animal studies of methicillin-resistant S. aureus (MRSA) bacteremia, prophylactic administration with the anti-ClfA monoclonal antibody was protective in mice against sepsis-induced mortality. Additionally, in a rabbit model of established MRSA infective endocarditis, tefibazumab therapy enhanced the efficacy of vancomycin by reducing the levels of S. aureus in the blood, vegetations, and organs. In a phase I study of healthy subjects, a dose of 20 mg/kg of body weight-maintained plasma levels of tefibazumab above 100 μg/ml, a concentration associated with maximal efficacy in animal models, for up to 21 day.
Mechanism of Action of Tefibazumab
Microbial surface components recognizing adhesive matrix molecules (MSCRAMM) proteins are a family of cell surface adhesins that recognize and specifically bind to distinct extracellular matrix components within host tissues or to serum-conditioned implanted biomaterials such as catheters, artificial joints, and vascular grafts. Once S. aureus successfully adheres to and colonizes host tissues, the expression of specific genes is altered, resulting in a phenotype that is more resistant to antibiotics. Therefore, interventions that impact early events in the infectious process may lead to an improved clinical outcome. Clumping factor A (ClfA) is an MSCRAMM protein expressed by S. aureus that promotes binding of fibrinogen and fibrin to the bacterial cell surface. The domain organization of ClfA is prototypic for the MSCRAMM subfamily of cell wall anchored staphylococcal proteins. The N-terminus contains a signal sequence followed by the ligand-binding A region that is composed of three subdomains N1, N2 and N3. C-terminal of the A region is the serine-aspartate repeat (Sdr) domain which can become glycosylated followed by the LPXTG motif and other features required for cell wall anchoring. The biological role of ClfA as a virulence factor and the therapeutic benefit of anti-ClfA antibodies have been evaluated in experimental animal models of septic arthritis and infective endocarditis. These data indicate that ClfA is a valid target for the development of novel immunotherapeutic agents. Tefibazumab protects against ClfA mediated destruction of host cells, preserving the human immune cells.
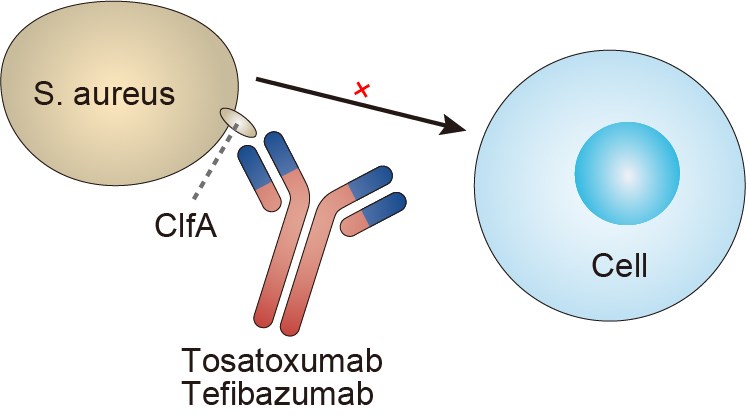
Fig.1 Mechanism of action of Tefibazumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

