Olendalizumab Overview
Introduction of Olendalizumab
Olendalizumab, as known as ALXN1007, is a proprietary antibody that targets the complement inflammatory pathway with potential immunomodulating and anti-inflammatory activities. It is a novel anti-inflammatory monoclonal antibody targeting complement protein C5a which has been evaluated in a Phase 2 trial for patients with acute GI-GVHD.
Mechanism of Action of Olendalizumab
Allogeneic hematopoietic cell transplantation (HCT) is one of the best curative modalities for patients with intermediate- and high-risk acute leukemia; approximately 3,500 patients receive allo-HCT for acute leukemia annually. The efficacy of this therapy is limited by the development of acute graft-versus-host disease (GVHD), which is measured by dysfunction in three organ systems: the skin, liver and gastrointestinal (GI) tract. Acute GVHD of the GI tract affects up to 60% of patients receiving allogeneic HCT, causing nausea, vomiting, anorexia, secretory diarrhea and, in more severe cases, severe abdominal pain and/or hemorrhage. Acute GVHD is often clinically indistinguishable from other causes of GI dysfunction such as conditioning regimen toxicity, infection, or medication effect. At present, GI-GVHD affects approximately 10 percent of patients who receive an allogeneic hematopoietic stem cell transplant or bone marrow transplant. Patients with severe, acute GI-GVHD have a 30 to 40 percent mortality rate within the first six months post-transplant. There are currently limited treatment options in acute GI-GVHD. The C5 gene encodes a component of the complement system, a part of the innate immune system that plays an important role in inflammation, host homeostasis, and host defense against pathogens. The encoded preproprotein is proteolytically processed to generate multiple protein products, including the C5 alpha chain, C5 beta chain, C5a anaphylatoxin and C5b. The C5 protein is comprised of the C5 alpha and beta chains, which are linked by a disulfide bridge. Cleavage of the alpha chain by a convertase enzyme results in the formation of the C5a anaphylatoxin, which possesses potent spasmogenic and chemotactic activity, and the C5b macromolecular cleavage product, a subunit of the membrane attack complex (MAC). Olendalizumab is a novel anti-inflammatory monoclonal antibody targeting complement protein C5a. Upon intravenous administration, anti-inflammatory antibody olendalizumab modulates the complement inflammatory pathway through binding to C5a.This inhibits C5a-mediated signal transduction, and results in the prevention and/or inhibition of both complement-mediated inflammation and cell destruction.
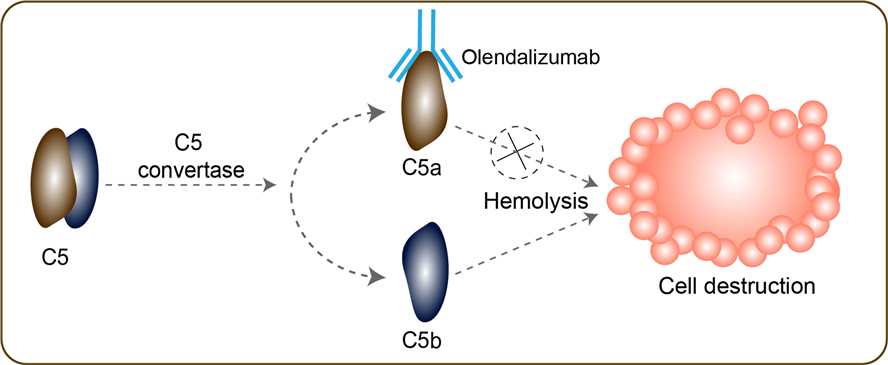
Fig 1. Mechanism of Action of Olendalizumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

