Dinutuximab Overview
Introduction of Dinutuximab
Dinutuximab (Unituxin), also known as ch14.18 (United Therapeutics Corporation [UTC]), is a novel chimeric, human-murine, anti-GD2 monoclonal antibody that underwent priority review by the Food and Drug Administration (FDA) and received approval with an orphan drug designation on March 10, 2015. It is the first monoclonal antibody specifically approved for use in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-2 (IL-2), and isotretinoin (13-cis-retinoic acid [RA]) for the maintenance treatment of pediatric patients with high-risk neuroblastoma who achieve at least a partial response to first-line multiagent, multimodality therapy.
Mechanism of Action of Dinutuximab
Tumor-associated gangliosides have emerged as promising targets for the development of monoclonal antibodies as immunotherapy for treating various cancers. Gangliosides (eg, GD2, GM2, GD3, NGcGM3, and OAcGD2) are glycosylated lipid molecules that belong to the glycosphingolipid class. GD2 is unique in that it is found in low levels on the surface of neurons, skin melanocytes, and peripheral sensory nerve fibers but is highly expressed on the cell surface of neuroblastomas. It has also been detected in melanomas, bone and soft-tissue sarcomas, and small-cell lung cancer. GD2 signaling has been shown to result in tumor growth and metastasis.
Dinutuximab binds to surface GD2 and signals antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), which results in tumor regression. Patients with high-risk neuroblastoma receive intense chemotherapy and undergo stem cell transplantation resulting in an immunosuppressed state. Granulocyte-macrophage colony stimulating factor (GM-CSF) and interleukin-2 (IL-2) increase production and activation of natural killer T cells, macrophages, and neutrophils from the patient’s own immune system to further enhance cytotoxicity against the neuroblastoma. Thus, dinutuximab is approved for administration with GM-CSF and IL-2.
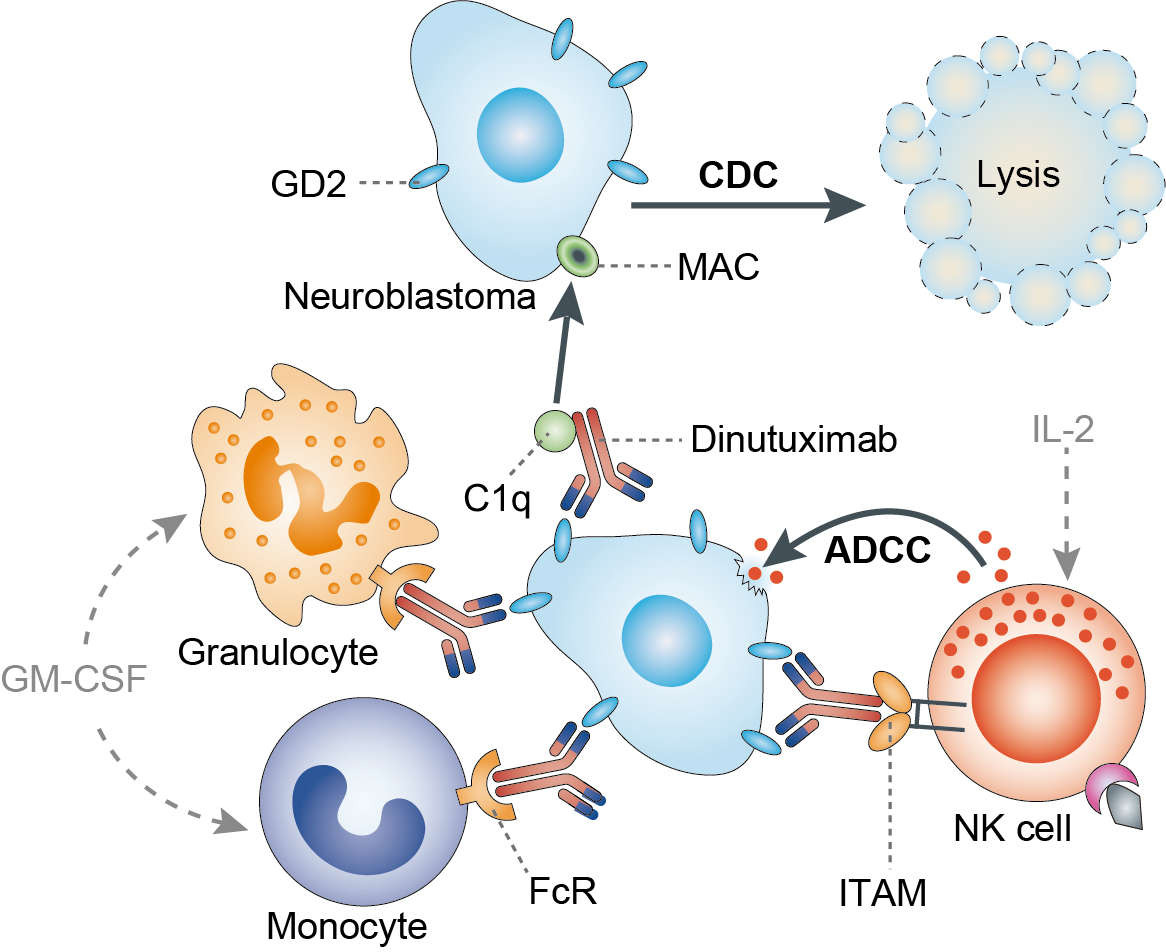 Fig.1 Mechanism of Action of Dinutuximab
Fig.1 Mechanism of Action of Dinutuximab
Table 1. Clinical Projects of Dinutuximab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03098030 | Active, not recruiting | Small Cell Lung Cancer | United Therapeutics | March 31, 2017 |
| NCT02169609 | Active, not recruiting | Neuroblastoma, Neoplasm Residual, Effects of Immunotherapy | Fundació Sant Joan de Déu | June 23, 2014 |
| NCT03332667 | Recruiting | Neuroblastoma | New Approaches to Neuroblastoma Therapy Consortium | November 6, 2017 |
| NCT03786783 | Recruiting | Ganglioneuroblastoma, High-Risk Neuroblastoma | National Cancer Institute (NCI) | December 26, 2018 |
| NCT02484443 | Active, not recruiting | Metastatic Malignant Neoplasm in the Lung, Metastatic Osteosarcoma, Recurrent Osteosarcoma | National Cancer Institute (NCI) | June 29, 2015 |
| NCT04211675 | Not yet recruiting | Relapsed Neuroblastoma, Refractory Neuroblastoma | Nationwide Children's Hospital | December 26, 2019 |
| NCT04253015 | Active, not recruiting | Neuroblastoma | EusaPharma (UK) Limited | February 5, 2020 |
| NCT03794349 | Recruiting | Ganglioneuroblastoma, Recurrent Neuroblastoma, Refractory Neuroblastoma | Children's Oncology Group | January 7, 2019 |
| NCT01711554 | Active, not recruiting | Recurrent Neuroblastoma, Refractory Neuroblastoma | National Cancer Institute (NCI) | October 22, 2012 |
| NCT02743429 | Recruiting | Neuroblastoma | University Medicine Greifswald | April 19, 2016 |
| NCT02573896 | Recruiting | Neuroblastoma | New Approaches to Neuroblastoma Therapy Consortium | October 12, 2015 |
| NCT02914405 | Recruiting | Neuroblastoma | University Hospital Southampton NHS Foundation Trust | September 26, 2016 |
Table 2. Approved Drugs of Dinutuximab**
| INN (trade name) | Therapeutic area | Dose | Strength | Route | Company | Marketing start | Market |
| Unituxin | Neuroblastoma | Solution | 3.5 mg / mL | Intravenous | UNITED THERAP | March 10, 2015 |
|
| Unituxin | Neuroblastoma | Solution | 3.5 mg / mL | Intravenous | United Therapeutics Europe Ltd | August 14, 2015 |
|
| Unituxin | Neuroblastoma | Solution | 3.5 mg / mL | Intravenous | United Therapeutics Corporation | May 1, 2019 |
|
References
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Dinutuximab
** Information presented in the table were collected from the following website:
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=125516
https://www.ema.europa.eu/en/medicines/human/EPAR/unituxin
https://health-products.canada.ca/dpd-bdpp/dispatch-repartition.do;jsessionid=E601E63913E00987ED0F9ADAD666968A
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

