Immunoblotting Protocol & Troubleshooting
Immunoblotting is primarily used for protein separation and identification. The brief steps are, isolating proteins by gel electrophoresis, transferring proteins to a membrane, using polyclonal or monoclonal antibodies to identify proteins by fluorescence or chemiluminescence detection.
Here we go over the immunoblotting protocols, including the reagents, solutions, procedures, and troubleshooting advice for typical issues. Our aim is to help you understand and explain the protocol processes and take full advantage of the detailed protocols for immunoblotting.
Solutions and Reagents
| Stages | Solutions and Reagents |
| Cell Lysis | Phosphate buffer saline (PBS), cell lysis buffer, protease inhibitors |
| SDS-PAGE | Stacking gel buffer, separating gel buffer, electrophoresis buffer, loading buffer, running buffer |
| Gel Transfer | Transfer buffer, blocking buffer |
| Immunoblotting | Primary antibody, secondary antibody, antibody dilution buffer, membrane washing buffer |
Workflow
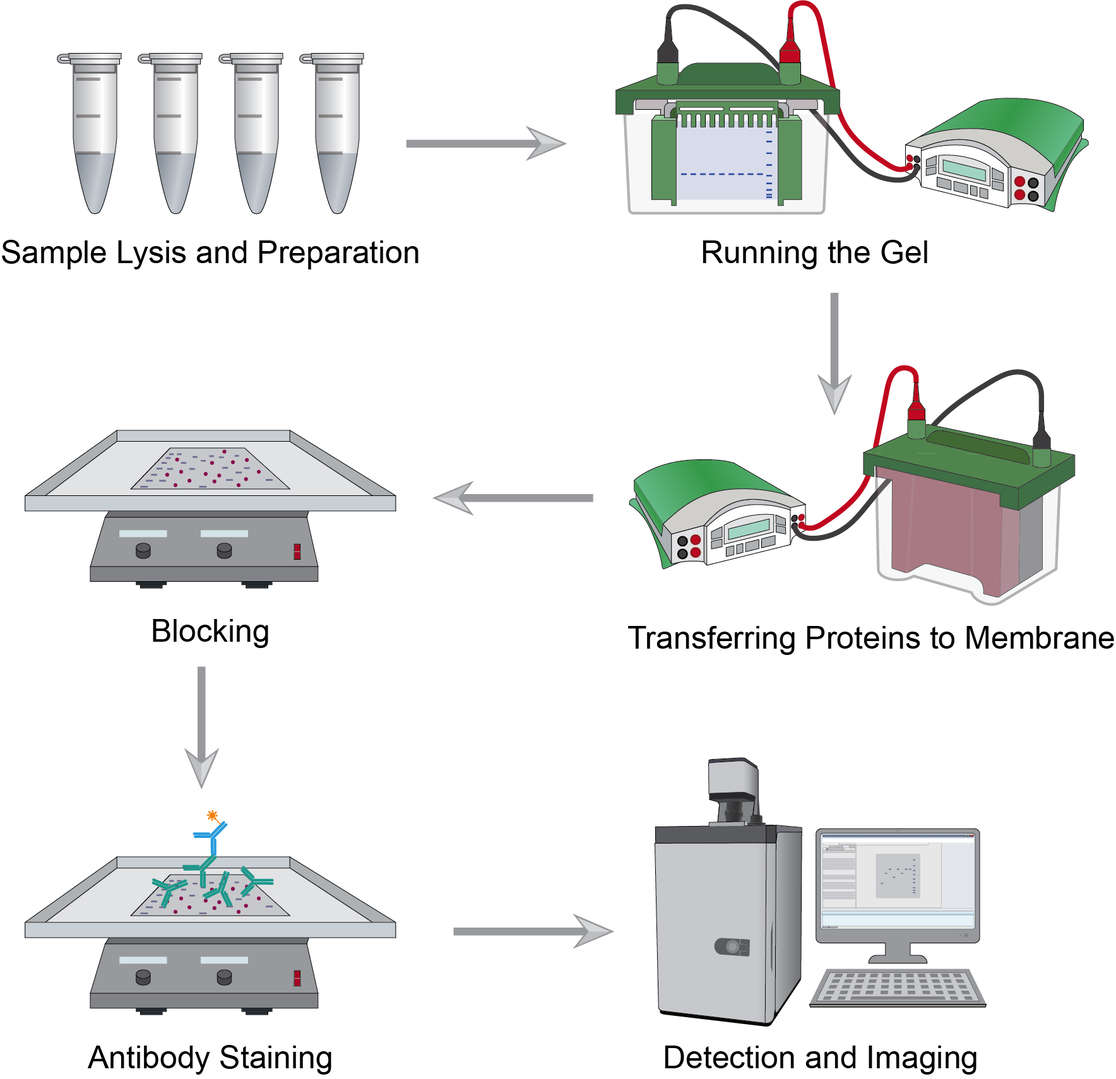 Workflow of Immunoblotting
Workflow of Immunoblotting
Proteins can be extracted from different sample types like cells and tissues that will go through ice-based procedures including cell washing, digestion, transfer, agitation, and centrifugation. Tissue samples should firstly be dissected, homogenized and lysed, and agitated, and the remaining steps are essentially the same as for cell samples. Determine the volume of lysate to be used for protein analysis, add sample buffer, and heat to reserve.
The percentage of gel depends on the size of the protein you are interested in, or you can use a gradient gel. Place the gel in the tank and fill with buffer, load the sample in the wells, and run at the appropriate voltage until the dye reaches the bottom of the gel.
Commonly used protein blotting membranes include nitrocellulose and polyvinylidene fluoride. After removing the gel, a transfer sandwich of gel and membrane is created and placed in the device for transfer. The specific running time and voltage should be proportional to the thickness of the gel.
Blocking can prevent non-specific binding of the antibody to the membrane. Place the membrane in the blotting vessel, add enough blocking solution to cover the entire membrane surface, and shake to incubate. This can help to reduce background noise while enhancing the target protein signal.
Primary antibody staining is a critical step. Incubate the membrane with the diluted primary antibodies, wash the membrane at the end of the incubation to remove excess primary antibodies, continue incubation with a good selection of secondary antibodies in the blocking buffer, and finally wash to remove excess secondary antibodies.
Select signal imaging reagents for detection, after which choose the machine of interest for imaging blotting and quantifying the outcomes.
Troubleshooting
Despite the simplicity of the immunoblotting, problems may still arise such as undetected or weak signals. For some of the problems that may occur, we provide the following troubleshooting guide.
- No band
- Antibody causes. If an unsuitable antibody is used, either a primary or secondary antibody, no band will show up; the concentration of the antibody should be appropriate, and no signal may show if it is quite low.
- Antigen causes. Problematic results may occur when the antigen concentration is very low or absent. Other sources of antigen can be used to confirm whether it is the sample that causes the issue, or something else, such as an antibody.
- Buffer solution causes. Make sure the buffer solution is not contaminated. "Overdose" wash time can also cause a reduced signal.
- Faint bands
- Antibody/antigen concentration causes. Low concentrations of antibodies or antigens may result in weak signals. Prolong the exposure time to help make the bands clearer.
- Incubation reagent causes. BSA or skim milk is typically used for antibody incubation and may mask the antigen, of which the amounts should be reduced when faint band occurs.
- Patchy and uneven spots
- Transferring causes. Air bubbles between the gel and the membrane, improper use of the shaker, or uneven mixing during the incubation process can cause spotty results.
- Blocking agent causes. It can also be the result of antibodies bound to the blocker. In such cases, an alternative blocker or filter the blocker should be adopted to remove the contaminant.
- Secondary antibody causes. Aggregation of secondary antibodies results in blotting on the blot. The secondary antibodies should be centrifuged and filtered to remove the aggregates.
- "Off course" bands
- Protease degradation causes. To avoid unexpected bands, a fresh sample kept on ice or another antibody can be applied.
- Gel causes. Uneven gel interface, uneven heating of the gel, unsuitable buffer concentration etc., can cause uneven bands which can be solved by optimizing the gel.
- Sample well causes. Poor polymerization around sample pores may cause skewed bands.
- Overdose of protein or antibody may lead to white bands.
- High background on the blot
- Antibody concentration causes. Antibody will bind to the PVDF membrane as a result of high antibody concentration.
- Gel causes. Overused gels can produce high background resolution. This can be improved by replacing the gel or increasing the wash time.
- Exposure causes. Overexposure can also cause this problem. Different exposure times can be examined to choose the best result.
Immunoblotting is a critical technique for protein detection. Creative Biolabs can be the support to secure your ultra-sensitive result. Please browse our immunoblotting protocols and choose from our complementary products and services.
Products with Tested Data
At Creative Biolabs, we are dedicated to providing high-quality antibodies for various research applications. Each product in our extensive range has been rigorously tested to ensure superior reliability and efficacy. To showcase the performance of our antibodies, we have conducted numerous experiments using Immunoblotting assay. Below, you will find a table listing a selection of our antibody products along with images from these experiments, demonstrating their proven reliability.
| Product Name | Catalog Number | Target | Image | Description |
|---|---|---|---|---|
| Human Anti-Apop U1-70K Recombinant Antibody (clone 20C4) | ZG-452C | Apop U1-70K |

|
Blot contains h apoptotic (left lane) and non apoptotic (right lane) Jurkat nuclear cell extract. Incubated with antibody fraction diluted in PBS containing 0,05% tween-20 and 5% non fat dry milk. |
| Mouse Anti-RPP20 Recombinant Antibody (clone 1F11) | ZG-406C | RPP20 |

|
Blot contains total cell extract of HEp2 cells. Incubated with antibody fraction (0.5 mg/ml) diluted 500X in PBS containing 0.05% tween-20 and 5% non fat dry milk. Left: washing under stringent conditions. Right: washing under less stringent conditions. |
| Mouse Anti-EXOSC2 Recombinant Antibody (clone 15B3) | ZG-407C | EXOSC2 |

|
Blot contains total cell extract of HEp2 cells. Incubated with antibody fraction (0.5 mg/ml) 500X diluted in PBS containing 0,05% tween-20 and 5% non fat dry milk. |
| Mouse Anti-EBFP Recombinant Antibody (clone 3A6) | ZG-411C | EBFP |

|
Blot contains total cell extract containing recombinant EGFP (predicted band size 27 kDa). Incubated with antibody fraction (0.5 mg/ml) 1000X diluted in PBS containing 0,05% tween-20 and 5% non fat dry milk. |
| Mouse Anti-IgY Recombinant Antibody (clone 08C) | ZG-420C | IgY |

|
Blot contains Na-alginate precipitated yolk IgY (predicted band size [heavy chain IgY] 70 kDa). Incubated with antibody fraction (culture supernatant) 10X diluted in PBS containing 0,05% tween-20 and 5% non fat dry milk. |
Reference
- Rasheed S, et al. Detailed Western Blotting (Immunoblotting) Protocol. 2022.
- Mahmood T and Yang PC. Western blot: technique, theory, and trouble shooting. N Am J Med Sci, 2012; 4(9): 429-434.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

