Costimulation Protocol & Troubleshooting
T cells usually require two signals to be fully activated. A first signal of antigen specificity is provided through the T-cell receptor (TCR). The TCR interacts with peptide-MHC molecules on the membrane of antigen-presenting cells (APCs). The second signal, the co-stimulatory signal, is non-antigen-specific. It is provided by the interaction between costimulatory molecules expressed on the APC membrane and T cells.
T cell stimulation is required for T cell proliferation, differentiation and survival. Creative Biolabs offers costimulation assays. Specific antibodies are used to mimic first and second signals in in vitro experiments to induce proliferative responses in resting lymphocytes. Below we briefly describe the costimulation protocol to study its modulation of T-cell function and to validate new targets for immunotherapy.
Solutions and Reagents
| Stages | Solutions and Reagents |
| Cell Preparation | Phosphate buffer (PBS), lymphocyte isolation solution, culture media |
| Costimulation | Anti-CD3 antibody, anti-CD28 antibody, washing buffer |
Costimulation Procedure
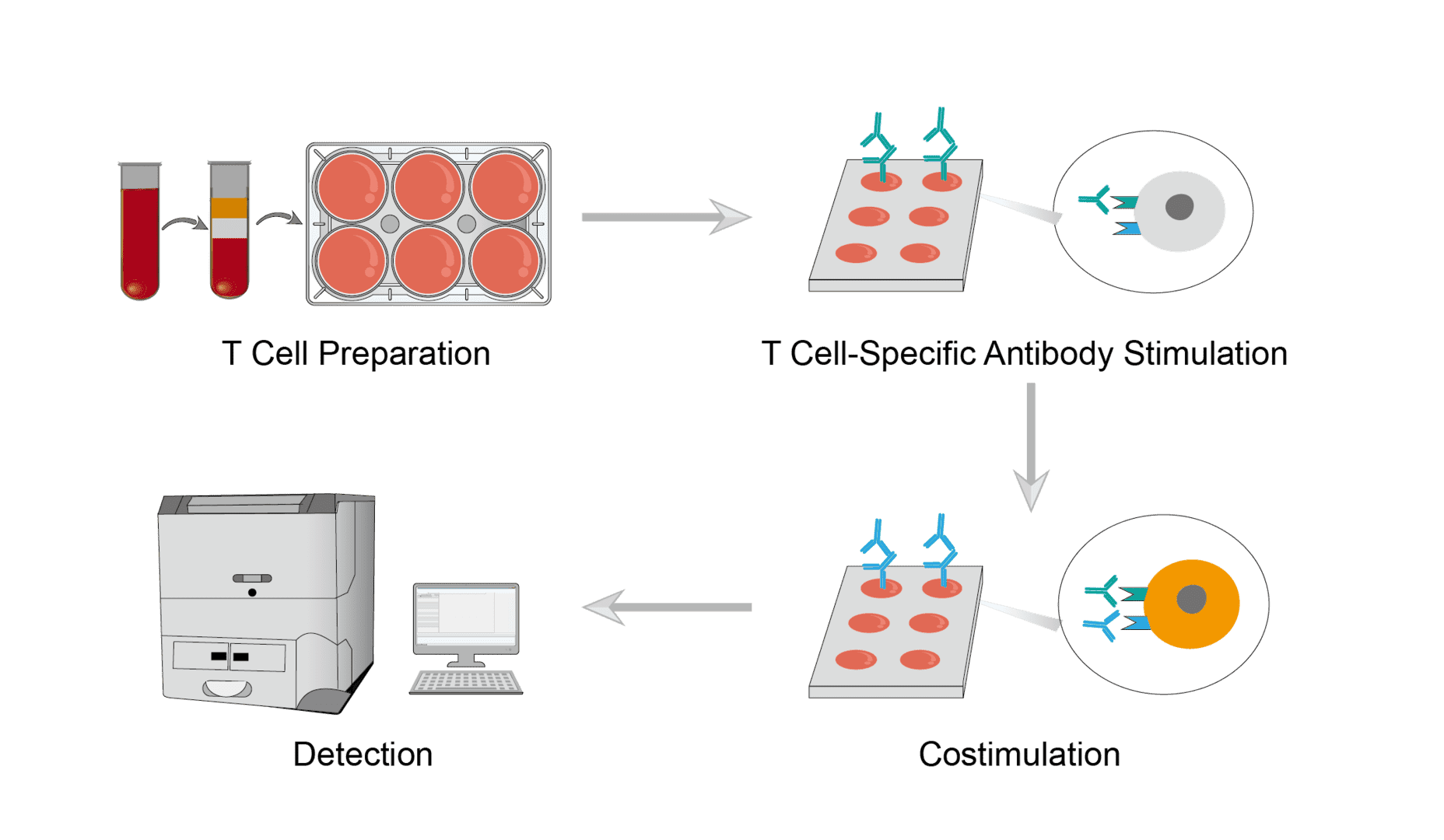
Collect target samples and isolate cells using lymphocyte isolation solution. Then collect the target cells and resuspend them in conditioned medium. Finally, count the cells and adjust the concentration of the cell suspension.
Add equal amounts of T cells to each well of the well plate, and set up positive control, negative control, and reference control groups respectively. Then add anti-CD3 antibodies that specifically recognize the surface molecules of T cells and incubate for an appropriate time. Finally, wash away the excess antibody solution.
Add costimulatory molecule-specific antibodies to the corresponding wells as a detection group. Costimulation with anti-CD3 antibody and anti-CD28 antibody is used as a positive control. A single stimulus is used as a reference control. T cells without any stimulation is used as a negative control. All reactions are incubated under consistent and appropriate conditions.
At the end of incubation, stain the cells to be tested with specific fluorescent dyes. Then place them in sample tubes for detection by flow cytometry. The value from the single anti-CD3 stimulus on each plate is taken as 0% costimulation value. And the value from the anti-CD3+ anti-CD28 stimulus is considered as a signal for 100% costimulation. The costimulatory effect to be assessed can be calculated from the standard curve.
Troubleshooting
Weak or no fluorescence signal
- Sample causes. First, use freshly isolated cells whenever possible, rather than frozen samples. Next, check if the cell count is high enough. You can use PBMC instead of T cells and simply remove non-T cell effects during the staining assay stage.
- Antibody causes. If your signal is weak, the antibody solution may be used too little. We recommend increasing the amount or concentration of antibody. You can also optimize antibody incubation time and temperature and consider using other steps to amplify the signal.
- Fluorescent dye causes. Choose the right fluorescent dye that will remove other non-T cell effects. And ensure that the fluorescence of the fluorescent dye does not fade during the assay.
- Reagent causes. Take care to ensure that all reagents and solutions are of good quality and stored according to the manufacturer's instructions.
High fluorescent signal
- Fluorescent dye causes. Match the appropriate fluorescent dye to the T cells.
- Washing causes. It may occur that excess antibodies are trapped in the samples. Wash the cells well after each antibody incubation step.
High background
- Non-specific binding causes. Antibody targeting to non-T cells results in high background. Non-specific receptors on cells are blocked with a blocking agent prior to antibody incubation.
- Washing causes. Increase the number of washes to ensure that excess antibody is removed.
If you encounter any problems, you can briefly describe your experiment in the inquiry form and we will have a professional technical team to answer your questions.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

