Clenoliximab Overview
Introduction of Clenoliximab
Clenoliximab (as known as IDEC-151) is a chimeric macaqueu-human monoclonal antibody of immunoglobulin (Ig) G4 isotype specific for the CD4 molecule on the surface of T lymphocytes. In addition, it inhibits antigen-induced T-cell proliferation, lymphokine release and helper T-cell functions. This drug, from Macaca irus and Homo sapiens, acts as an immunomodulator and has been investigated for the treatment of rheumatoid arthritis (RA). It has been studied in patients with rheumatoid arthritis in which T cell activation orchestrates inflammation and tissue damage. In single-dose studies in patients with active RA, clenoliximab caused dose- and time-dependent CD4 coating and down-modulation with no significant depletion of CD4+ T cells and the data suggested that downmodulation is caused by antibody-mediated stripping of CD4 from the lymphocyte cell surface. Clenoliximab has the same antigen-combining site as keliximab, an IgG1 monoclonal antibody that showed promise in the treatment of RA in phase II/III clinical trials. However, clenoliximab has greatly reduced Fc receptor affinity as a result of the IgG4 isotype and several key amino acid substitutions in the constant heavy chain domain 2.
Mechanism of Action of Clenoliximab
CD4 is a surface antigen found on several immune cell types, primarily on the helper/initiator subset of T lymphocytes. On T helper cells, CD4 acts in conjunction with the T cell receptor as an accessory protein by binding MHC Class II antigens (primarily on antigen presenting cells) and subtending protein kinase p56Ick signal transduction. Because of the central role of T helper cells as regulators of immune function, they are believed to play an important role in the pathogenesis of several immunologically mediated diseases, e.g., asthma and rheumatoid arthritis (RA), and anti-CD4 monoclonal antibodies are being explored as a therapeutic modality in these diseases. Clenoliximab is a macaque-human chimeric monoclonal antibody (immunoglobulin G4) specific for the CD4 molecule on the surface of T lymphocytes. It is also a modified IgG4 derivative of keliximab designed to minimize cell depletion. The γ4 constant region contains two amino acid substitutions relative to native γ4. Substitution of glutamic acid for leucine at residue 235 in the CH2 region reduces residual FcγRI binding, and substitution of proline for serine at residue 228 in the hinge region, stabilizes disulfide bond interactions between the heavy chains of the mAb. Clenoliximab can mediate in vivo immunomodulatory effects through 1) removal of CD4+ T cells by apoptosis or complement-mediated lysis of mAb-coated CD4+ T cells; 2) receptor modulation caused by internalization or stripping of the CD4 receptor from the cell surface; and 3) inhibition of T cell activation by antagonism of CD4-MHC class II interactions, possibly leading to induction of anergy. Although this drug can act through these mechanisms, the Fc-dependent activities of T cell depletion and receptor modulation was strongly reduced for clenoliximab.
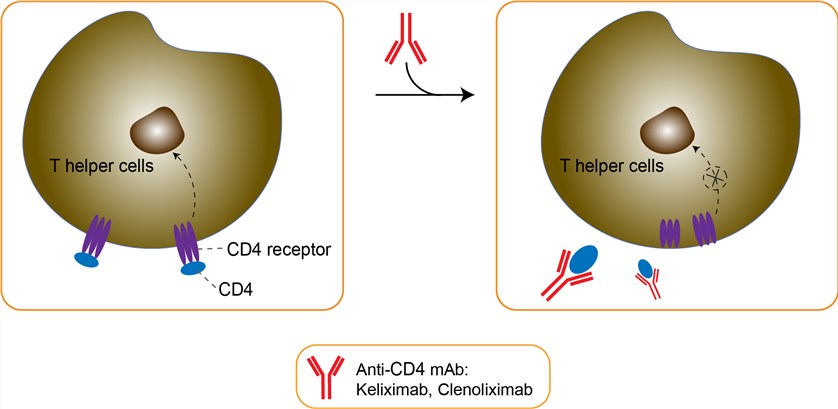
Fig 1. Mechanism of Action of Clenoliximab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



