Bapineuzumab Overview
Introduction of Bapineuzumab
Bapineuzumab is a humanized form of the murine mAb 3D6, targeting the N-terminus of Aβ, which, as previously mentioned, was shown in Tg mice to bind to plaques and induce Fc-receptor-mediated microglial phagocytosis. This treatment, first developed by Élan and Wyeth and then Janssen and Pfizer, advanced into phase 1, 2, and 3 trials. The overall safety and tolerance of bapineuzumab were established in phase 1. In phase 2 studies in patients with mild to moderate AD, this drug reduced phosphorylated tau (p-tau) protein in cerebrospinal fluid (CSF) and 11C-Pittsburgh compound B (PiB) average uptake visualized by positron emission tomography (PET). The findings provided a rationale for conducting separate trials in apolipoprotein E (ApoE) ε4 allele carriers and noncarriers and for limiting the bapineuzumab dose in carriers to minimize risk of amyloid-related imaging abnormalities with edema or effusion. A number of phase 3 trials were initiated between 2007 and 2009. However, four of these were terminated following failure in the first two completed trials due to no significant treatment effect on cognitive outcomes among either ApoE4 carriers or noncarriers.
Mechanism of Action of Bapineuzumab
Alzheimer's disease, a neurodegenerative disease resulting in progressive dementia, is characterized by neuropathological changes that include intraneuronal neurofibrillary tangles and extracellular neuritic plaques. The predominant component of plaques is the amyloid-beta (Aβ) peptide. Ever since the discovery that Aβ is the major constituent of amyloid plaques in AD and that familial AD results from mutations in the gene for amyloid precursor protein (APP) or in genes responsible for processing APP to Aβ, there has been a push to develop anti-amyloid therapeutics. Driven in part by the success of antibody therapies to target and destroy tumor antigens in neoplastic disease, and by the absence of competition from less costly small molecules, immunotherapy against Aβ emerged as the industry’s best hope for the first marketable disease-modifying agent for AD. Bapineuzumab is a humanized N-terminal–specific anti-Aβ monoclonal antibody in clinical development for the treatment of Alzheimer's disease. The rationale of this passive immunotherapy approach is that antibody binding will clear excess Aβ. Bapineuzumab is an IgG1 antibody that binds fibrillar and soluble Aβ and activates microglial phagocytosis and cytokine production. A small fraction of peripherally administered antibody enters the CNS of PDAPP and other mouse models of Aβ amyloidosis. The antibody was shown to bind to amyloid plaques, lower plaque burden, improve measures of synaptotoxicity, and improve performance on mouse behavioral assays.
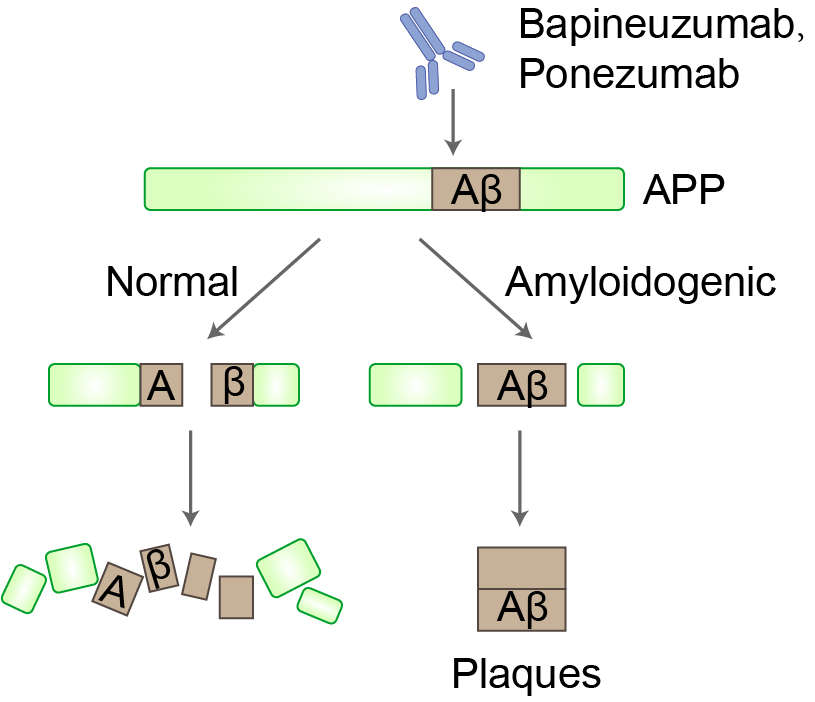 Fig 1. Mechanism of Action of Edobacomab
Fig 1. Mechanism of Action of Edobacomab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

