Siplizumab Overview
Introduction of Siplizumab
Siplizumab is a humanized immunoglobulin G1κ (IgG1κ) monoclonal antibody (mAb) that binds to the human CD2 receptor found on T cells and natural killer (NK) cells. CD2 is a transmembrane glycoprotein with an important role in both T-cell and NK-cell functions.
Mechanism of Action of Siplizumab
The interaction between leukocyte-function-associated antigen type 3 on antigen-presenting cells and CD2 serves as a co-stimulatory signal for T-cell activation and proliferation. CD2 also mediates the adhesion between activated T cells or NK cells and target cells. The receptor for CD2 is LFA-3 (CD58). CD2/LFA-3 engagement mediates cell adhesion to antigen-presenting cell (APC) that enhances antigen recognition and subsequent T-cell activation. Activated T cells appear to be preferentially targeted for antibody (anti-CD2) -dependent cellular cytotoxicity (ADCC), as the avidity and number of CD2 molecules are upregulated after activation. Treatment of mixed lymphocyte reaction with siplizumab revealed pronounced depletion of T cells and NK cells, suggesting that deletion of activated cells is at least partially responsible for the immunosuppressive effect of siplizumab. These in vitro studies further suggested that the depletion of activated T cells occurs via NK cell-mediated cytotoxic mechanisms. Data from a transgenic human CD2 mouse model also showed that siplizumab at high doses can deplete lymphocytes, whereas lower doses induce T-cell hypo responsiveness. In vitro studies have also demonstrated that siplizumab induces alloantigen hypo responsiveness, similar to its precursor molecule, BTI-322. Thus, although these effects need to be confirmed in humans, siplizumab may not only prevent the activation of T cells but may also elicit a state of alloantigen-specific unresponsiveness. If a clinical human correlate of the hypo responsiveness shown in the mouse is also observed in psoriasis, a positive impact on the pathophysiology of the disease may be realized. Preliminary data from clinical studies of siplizumab suggest that the agent exhibits an acceptable safety profile when administered as single intravenous (I.V.) infusions or multiple I.V. and subcutaneous (S.C.) escalating dose levels. Dose-dependent reductions in absolute lymphocyte counts (ALCs) were observed in these studies. In a few patients, ALCs and CD4 counts remained low throughout an extended follow-up period.
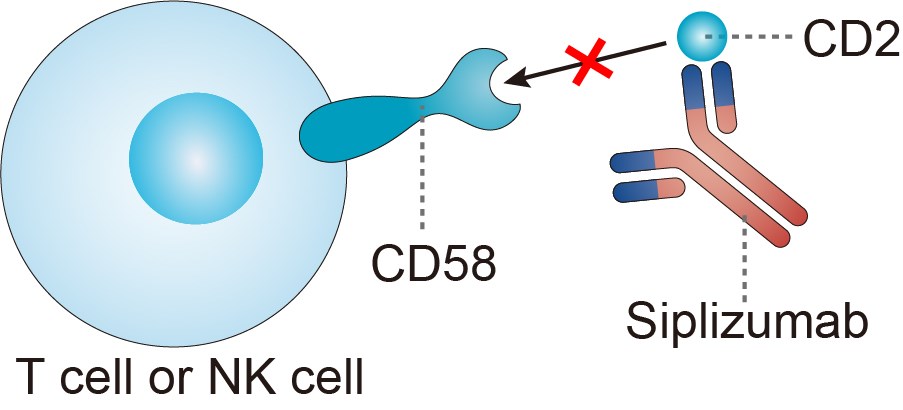
Fig.1 Mechanism of action of Siplizumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

