In Situ Hybridization Protocol & Troubleshooting
In situ hybridization (ISH) is a technique that uses nucleotide probes targeting the sequence of interest to hybridize to locate and visualize specific sequences. This method allows RNA or DNA molecules to be seen in a fixed cell or tissue. ISH can be widely used to study infectious agents, cancer or developmental biology.
ISH is a powerful technology. Creative Biolabs provides you with general procedures and tips for performing ISH, specifically including protocols, material support and a set of useful troubleshooting tips. Any laboratory scientist can easily use it for fast and efficient detection of gene of interest expression and mRNA location.
Solutions and Reagents
| Stages | Solutions and Reagents |
| Sample Preparation | Washing buffer, fixative, coating solution |
| Pretreatment | Protease, hydrochloric solution, blocking buffer, washing buffer |
| Hybridization | Hybridization solution, probe solution, washing solutions |
In Situ Hybridization Procedure
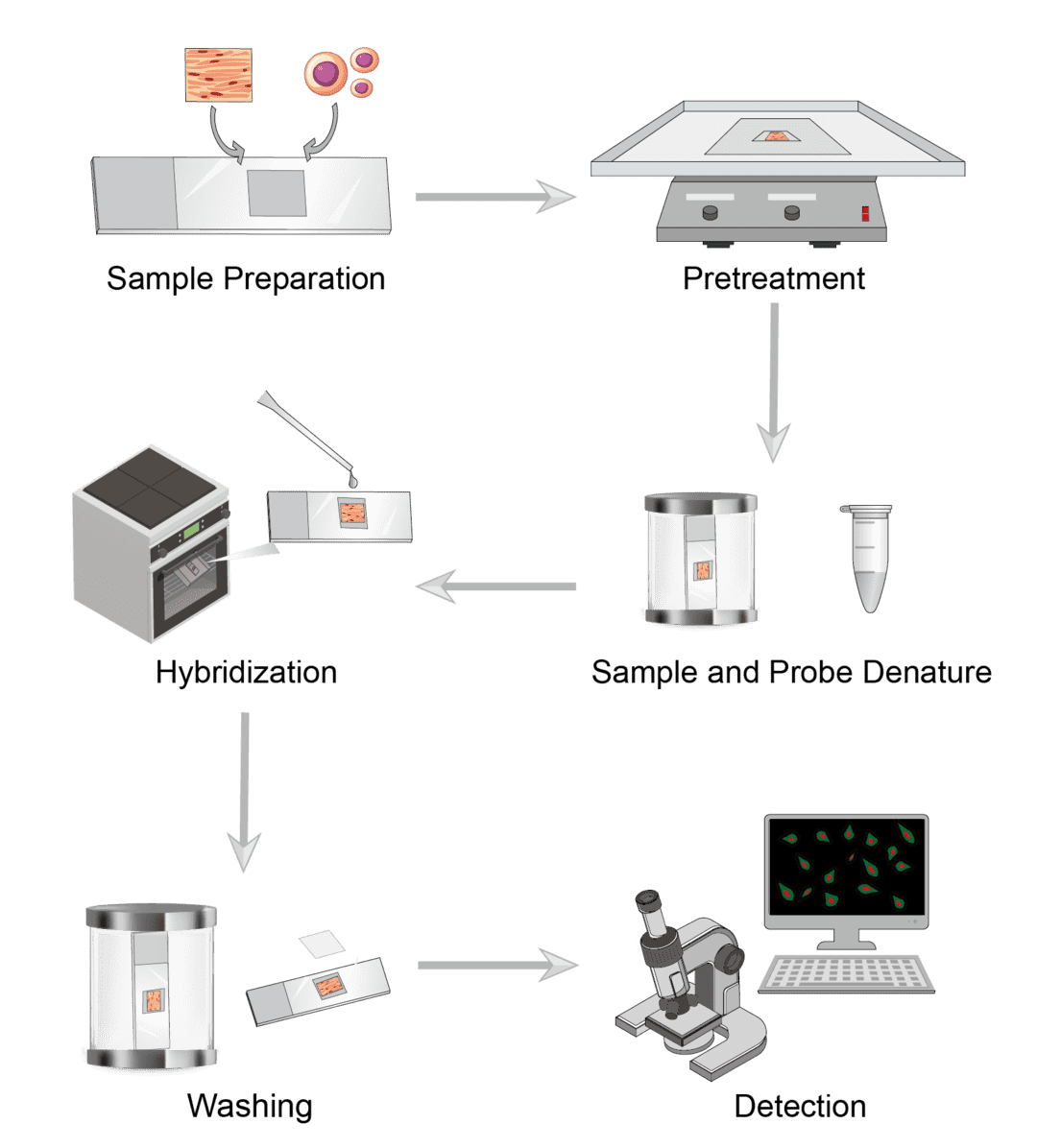
Common sample types for ISH include cells and tissues. Cell samples may include smears, cell debris, cell pellets, etc. Tissue sections may include frozen, paraffin sections, etc. Select the appropriate fixative for the sample. Ideally, ISH fixation should preserve both RNA and DNA as well as tissue morphology. Pre-treat the slide with a suitable coating solution to ensure that the tissue section adheres to the glass slide.
A series of preprocessing steps prior to hybridization can improve the efficiency of hybridization and reduce non-specific background staining. It is necessary to first permeabilize the sample. Treat with protease to increase the accessibility of the target by digesting the proteins surrounding the target nucleic acid. Combining this with other unmasking techniques such as treatment with sodium bisulfite, sodium thiocyanate or hydrochloric acid increases the hybridization signal. Certain tissues have endogenous biotin or alkaline phosphatase (AP). Use blocking agents to inhibit non-specific binding.
Specificity, sensitivity, ease of tissue penetration, stability of the hybrid and reproducibility of the technique must be considered when selecting the best probe for ISH. Select labeling molecules with relatively high specific activity and morphological resolution, such as biotin, fluorescein, and other non-isotopic labels.
Dilute the probe with the hybridization solution, heat to denature the probe and cool it immediately on ice to prevent re-annealing. At the desired hybridization temperature, add the diluted probe to the slide covering the entire sample and incubate in a humidified hybridization chamber. Optimize the hybridization temperature based on the sequence of the probe used and the sample type.
After hybridization, remove unbound probes or probes that bind incompletely matched sequences with washing solutions. Perform washes under stringent conditions close to where hybridization occurs. Control solution parameters such as temperature, salt and detergent concentration to eliminate non-specific interactions.
We can demonstrate the presence of a probe-target hybrid by detecting the probe label molecule. If you use isotopically labeled probes, they can be detected by photographic film or photographic emulsion. If you use directly fluorochrome or enzyme-labeled probes, they can be detected directly by instant fluorescence microscopy or by incubation in a substrate solution, respectively.
Troubleshooting
Are you having trouble with your experiments? We are committed to achieving success in your experiments. Check out our troubleshooting tips for common problems in ISH experiments.
No or weak signals
- Sample causes. Inadequate digestion and over-fixation of tissue/cell samples during the sample pretreatment step can result in decreased signal intensity. You need to verify the digestion temperature and time, and fixation length to optimize the conditions before reaching hybridization optimal.
- Probe causes. First check if the probe matches the coupling and subsequently the enzyme substrate. Next you can check the probe quality for good activity and resolution as well as preservation. Repeat the test reaction signal using different concentrations and different probe volumes to ensure that the probe volume and concentration used are optimal conditions.
- Hybridization causes. Please verify that the hybridization time, temperature, hybridization buffer and other parameters match. You can take alternative hybridization buffer and optimize hybridization conditions to solve the problem.
- Microscope causes. Check all items in the microscope kit to ensure that the problem was not caused by an assembly error. It is worth noting to determine if the filter set used to view the slide is appropriate. If they do not fit then they need to be replaced with the recommended filters.
High background
- Probe causes. Background staining in ISH may be caused by the nucleic acid probes used. If your probe contains a large number of repetitive sequences, this may increase background staining. You can prevent the probe from binding to these repetitive sequences by adding a suitable blocker to the hybridization process.
- Washing causes. High background staining may occur if the stringent washing step is not sufficient. Please follow the washing procedure strictly to ensure that the washing solution, washing temperature, and washing length are appropriate for adequate washing.
- Counterstaining causes. When there is background staining, the reaction should be stopped by rinsing the slide with distilled water. You can use a light counterstaining agent if needed.
- Microscope causes. We recommend following the staining reaction under a microscope rather than by the naked eye, as this may result in higher than necessary background staining.
Tissue loss or tissue morphology degraded
- Slide causes. Unsuitable slides may cause sample loss, and we recommend using positively charged slides. Please pay attention to the baking temperature of the slides during hybridization. As well as when removing coverslips after hybridization, tissue sections can be easily torn. You can soak the slides in washing buffer to give more time to remove the coverslips.
- Fixation causes. Insufficient fixation leads to sample loss or degradation. You can optimize fixation by changing fixatives or increasing the length of fixation.
- Pretreatment causes. Excessive pretreatment, such as digestion, leads to morphological degradation of the sample. Please optimize tissue digestion time and temperature to ensure that tissues are not over-processed.
- Hybridization causes. During hybridization, excessive denaturation will lead to loss of sequence. Denaturation time and ISH hybridization time need to be precisely controlled.
Signal strength variation
- Coverslip causes. This may be caused by air bubbles under the coverslip and uneven distribution of the probe on the slide. Repeat the test on adjacent sections of the same tissue block to ensure that there are no air bubbles under the coverslip. When mounting, place the coverslip in contact with the probe solution first to ensure that no air bubbles are generated.
We can complete the entire ISH protocol quickly, producing highly consistent results. We can likewise develop and validate assays to meet your specific needs. For all questions and concerns regarding any of our products and services, our technical support team is ready to assist you.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

