Certolizumab Pegol Overview
Introduction of Certolizumab Pegol
Certolizumab pegol (CDP870, tradename Cimzia) is a recombinant, humanized, pegylated monoclonal antibody (mAb) formed with a humanized Fab fragment of 50 kDa, from an IgG 1 isotype, fused to a 40 kDa polyethylene glycol moiety replacing the Fc antibody region. It was developed and manufactured by UCB Pharma. Targeting the tumor necrosis factor-alpha (TNFα), it has been approved by the U.S. Food and Drug Administration (FDA) to treat adults with Crohn’s disease (CD), rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS), non-radiographic axial spondyloarthritis (nr-axSpA), and plaque psoriasis (PsO).
Mechanism of Action of Certolizumab Pegol
Certolizumab pegol is a novel Fc-free, PEGylated, anti-TNFα monoclonal antibody. The parent antibody was selected from a screen of hybridomas for human TNFα binding. The complementarity determining regions (CDRs) from the murine antibody were then inserted into a human Fab’ IgG framework, along with several other framework residues of the variable domain that were essential for maintenance of affinity. The certolizumab Fab’ was subsequently PEGylated via the site-specific attachment of a 40 kDa polyethylene glycol (PEG) moiety. Certolizumab pegol binds and neutralizes both soluble and transmembrane TNFα and inhibits signaling through both the p55 and p75 TNFα receptors in vitro. Certolizumab pegol differs from the other TNFα-inhibitors in its lack of an Fc region, which minimizes potential Fc-mediated effects such as complement-dependent cytotoxicity (CDC) or antibody dependent cell-mediated cytotoxicity (ADCC). In vitro studies have shown that certolizumab pegol does not induce CDC and ADCC. In vitro studies have also demonstrated that certolizumab pegol, unlike other TNFα-inhibitors, does not cause activated peripheral blood lymphocytes to undergo apoptosis and that it inhibits lipopolysaccharide-induced cytokine production to a greater extent than other TNFα-inhibitors. The lack of an Fc region may also be a factor in the prevention of active transfer of certolizumab pegol across the placenta during pregnancy. Certolizumab pegol also differs from the other TNFα-inhibitors as it is PEGylated. PEGylation is widely used to improve drug pharmacokinetics and bioavailability. It has been demonstrated that PEGylation significantly increases the circulating half-life of Fab’ molecules, permitting a minimum dosing interval of 2 weeks for certolizumab pegol. Biofluorescence imaging in arthritic mice has shown that certolizumab pegol preferentially penetrates inflamed tissue compared to non-inflamed tissue, and does so to a greater extent than infliximab. Furthermore, the persistence of drug in the inflamed tissue was more prolonged for certolizumab pegol compared to infliximab. These phenomena are most likely due to the PEGylation of the molecule, although the persistence in the tissue could also be due to the lack of Fc receptor-mediated recycling of the Fab’. These features of certolizumab pegol may be relevant to its action in RA. In animal models, the 40 kDa PEG moiety is cleaved from the Fab’ and is excreted intact via the renal route. It was found that PEG is rapidly excreted once cleaved from the Fab’ portion of the molecule. The majority of PEG is estimated to be removed by the kidneys.
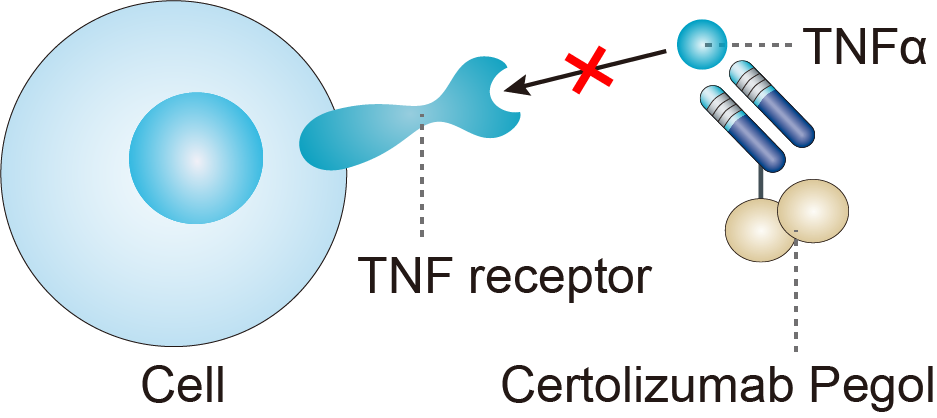 Fig.1 Mechanism of Action of Certolizumab Pegol
Fig.1 Mechanism of Action of Certolizumab Pegol
Table 1. Clinical Projects of Certolizumab Pegol *
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT04053881 | Recruiting | Plaque Psoriasis | UCB Biopharma S.P.R.L. | August 13, 2019 |
| NCT03215277 | Active, not recruiting | Ankylosing Spondylitis | UCB Biopharma S.P.R.L. | July 12, 2017 |
| NCT01550003 | Active, not recruiting | Polyarticular-course Juvenile Idiopathic Arthritis (JIA) | UCB BIOSCIENCES GmbH | March 9, 2012 |
| NCT00844285 | Active, not recruiting | Crohn's Disease | UCB Pharma | February 16, 2009 |
| NCT02552212 | Active, not recruiting | Axial Spondyloarthritis, Nonradiographic Axial Spondyloarthritis, Nr-axSpA | UCB BIOSCIENCES GmbH | September 17, 2015 |
| NCT01095393 | Enrolling by invitation | Rheumatoid Arthritis | UCB Pharma | March 30, 2010 |
| NCT01090154 | Active, not recruiting | Ulcerative Colitis | University of Washington | March 19, 2010 |
| NCT02120807 | Active, not recruiting | Stage IV Lung Adenocarcinoma | Memorial Sloan Kettering Cancer Center | April 23, 2014 |
| NCT03152058 | Recruiting | High Risk Pregnancy, Pregnancy Complications, Antiphospholipid Syndrome in Pregnancy, Lupus Anticoagulant Disorder | Ware Branch | May 12, 2017 |
| NCT01764321 | Active, not recruiting | Rheumatoid Arthritis | UCB Pharma SA | January 9, 2013 |
| NCT01797224 | Recruiting | Crohn's Disease, Rheumatoid Arthritis, Ankylosing Spondylitis, Psoriatic Arthritis, Psoriasis | University of California, San Diego | February 22, 2013 |
Table 2. Approved Drugs of Certolizumab Pegol**
| INN (trade name) | Therapeutic area | Dose | Strength | Route | Company | Marketing start | Market |
| Cimzia | Crohn’s Disease, Rheumatoid Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, Non-radiographic Axial Spondyloarthritis, Plaque Psoriasis | Solution | 200 mg / mL | Subcutaneous | UCB Inc. | April 22, 2008 |

|
| Cimzia | Arthritis, Rheumatoid | Solution | 200 mg / mL | Subcutaneous | UCB Pharma SA | October 1, 2009 |

|
| Cimzia | Rheumatoid Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, Non-radiographic Axial Spondyloarthritis, Plaque Psoriasis | Solution | 200 mg / mL | Subcutaneous | UCB Japan Co. Ltd. | December 25, 2012 |

|
| Cimzia | Rheumatoid Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, Non-radiographic Axial Spondyloarthritis, Plaque Psoriasis | Solution | 200 mg / mL | Subcutaneous | UCB Australia Pty Ltd T/A UCB Pharma Division of UCB Australia | January 20, 2010 |

|
| Cimzia | Rheumatoid Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, Non-radiographic Axial Spondyloarthritis, Plaque Psoriasis | Solution | 3.5 mg / mL | Subcutaneous | UCB Canada Inc. | August 31, 2009 |

|
References
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Certolizumab%20Pegol
** Information presented in the table were collected from the following website:
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=125160
https://www.ema.europa.eu/en/medicines/human/EPAR/cimzia-0
https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=81737
http://search.tga.gov.au/s/search.html?collection=tga-artg&profile=record&meta_i=154726
https://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/3999437
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

