Immunohistochemistry-Frozen Protocol & Troubleshooting
The two main methods for performing immunohistochemistry include paraffin-embedded sections and frozen sections. Frozen sections are simple, and quick to prepare. Frozen sections are mostly used for fresh tissues, formaldehyde-fixed tissues, refrigerator frozen tissues, etc.
We describe general procedural guidelines for system preparation and immunohistochemistry-frozen (IHC-Fr), and the client can determine the application protocol based on the target antigen. In addition to the protocols used for IHC-Fr, we also list some useful tips and troubleshooting guides.
Solutions and Reagents
| Stages | Solutions and Reagents |
| Section Preparation | Freeze embedding medium, PBS, fixative, protease inhibitors, blocking buffer |
| Antibody Staining | Blocking buffer, primary antibody, labeled secondary antibody, dilution buffer, PBS, incubation buffer, chromogen |
| Mounting | Mounting solution, PBS |
Immunohistochemistry Procedure
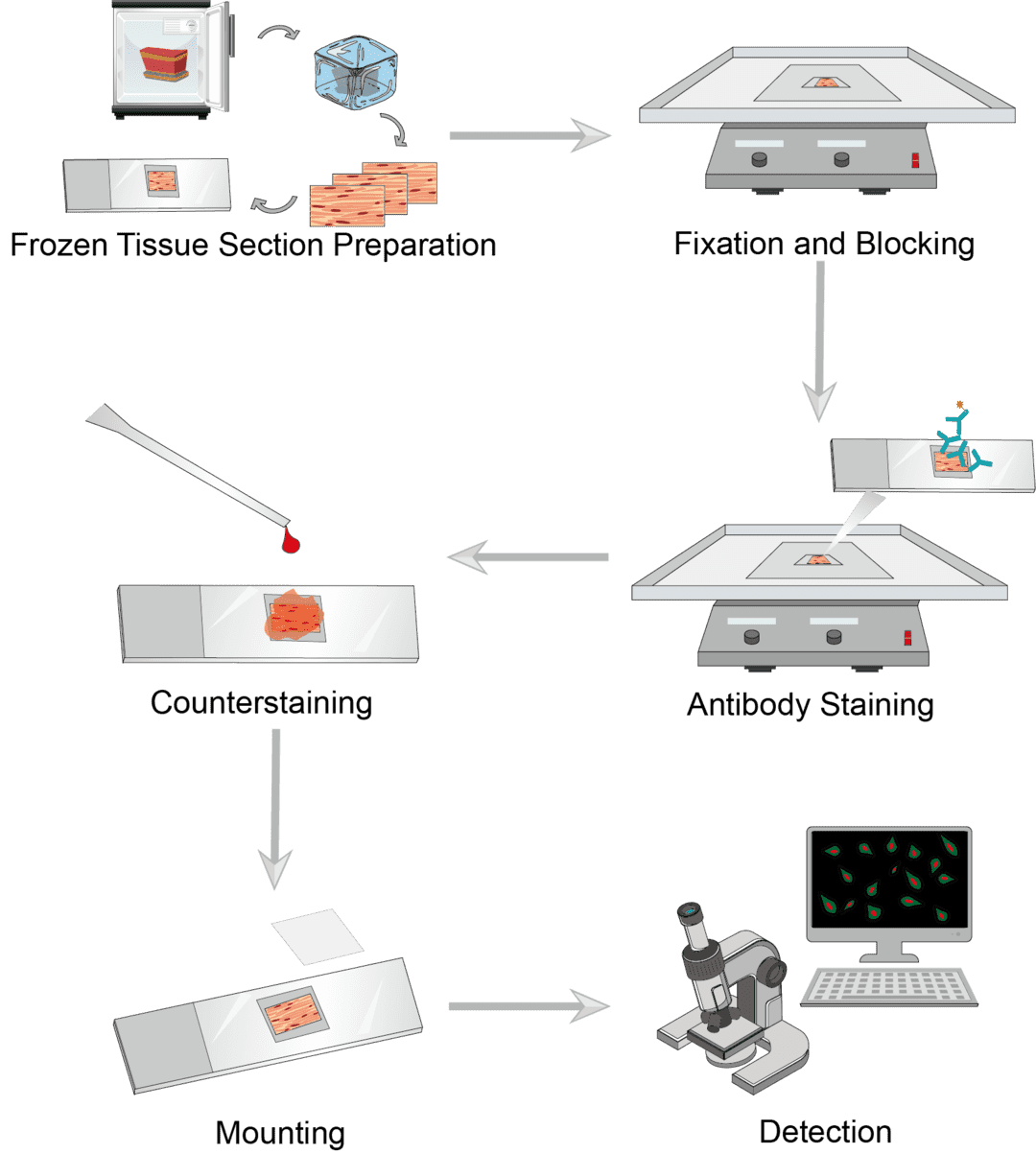
Prepare freshly dissected tissue blocks on a tissue substrate and cover the entire block with the frozen embedding medium. Freeze the tissue block in liquid nitrogen, transfer the frozen tissue block to a frozen sectioning machine, and slice the frozen tissue block to the desired thickness. Place the tissue sections on slides and dry the tissue sections for use.
Fix the tissue section with a suitable fixative and immerse the slide in the pre-cooled fixative. Then pour off the fixative and rinse the slides. After fixation is complete, the slides are incubated in the blocking buffer to block the non-specific binding of tissue immunoglobulins. At the end of the incubation, the slide is rinsed to drain the blocking buffer.
For direct labeling, dilute the primary antibody appropriately and transfer the slide to the incubation buffer containing the primary antibody for incubation. Then wash the slides. For indirect assays, transfer the sections to the incubation buffer containing the secondary antibody and continue incubation. Wash the slide after completion to remove excess solution. Add the appropriate dilution of chromogenic agent to the slides and incubate away from light.
The counterstaining step is optional. Counterstaining provides contrast with antibody staining to better distinguish the target signal. Select the ideal counterstains, incubate the sections, and rinse them upon completion.
After staining, use the appropriate sealing solution to mount coverslips and seal the samples. Mounted slides can be stored permanently at room temperature. Observe the color of the antibody stain in the tissue section under the microscope.
Troubleshooting
The following troubleshooting guide is intended to explain the causes and possible solutions to common problems observed in IHC-Fr staining. If you have any questions, please refer to our extensive troubleshooting guide.
Tissue section problem
- Freezing artifact problem. Tissues that generally contain water show more of these artifacts. Tissue should be frozen quickly when making samples, and tissue specimens should not be in salt water before freezing.
- Uneven tissue embedding problem. The uneven surface of the tissue section may result in the lost of important information. You should ensure that the freezing blade is sharp enough and adjust the cutting speed to cut thicker sections.
- Tissue section detachment problem. Use positively charged or coated slides, or replace them with new slides. Allow frozen sections to air dry for at least 30 minutes prior to fixation and then for another 30 minutes prior to immunostaining. During the fixation step, you can change the fixative or increase the fixation time.
No or inadequate staining
- Antigen causes. If the test slide is inadequately stained or unstained, while the positive control slide is adequately stained, the antigen may be the problem. The antigen is not present in the test tissue or is present but at a level below the limit of detection. Consider using an amplification procedure or increasing the primary antibody concentration, incubation time, or temperature.
- Antibody causes. If the positive control is weak or unstained and the test slide is also weak or unstained, the antibody may be the cause. Check the antibody, including conditions such as dilution concentration, incubation time, and temperature. Pay attention to check if the secondary antibody and primary antibody are compatible because the test requires the use of a secondary antibody that will interact with the primary antibody.
- Reagent causes. Check and make sure that the buffer solution is not contaminated, and that the wash time is appropriate as a long-time wash can cause a reduced signal. It is necessary to check the relevant reagents such as buffers and chromogenic agents, and expiration dates, storage parameters, pH, and order of use are all included.
High background
- Section causes. Consider first if the tissue section is too thick and thinner sections can be prepared. Then check if sections are adequately fixed or if necrosis and autolysis are present. If any of these conditions are present, avoid sampling necrotic areas and ensure that the tissue is properly fixed. As well, inappropriate kinds of or the excess use of section adhesives can affect the background. Throughout the process, check and ensure that tissue sections are not dry.
- Blocking causes. Possible reasons for this are inadequate blocking of endogenous enzyme activity, biotin, or protein during the blocking phase. You can increase the concentration of the blocking agent or use a different blocking agent. In addition, you need to be aware that the blocking buffer should be made from a blocking serum of the same species.
- Primary antibody causes. Improper antibody concentration causes high background. You can re-titrate the primary antibody and select the appropriate concentration. Check the primary antibody incubation time, too long time can also cause this problem. You can shorten the incubation time. Clean sections and no antibody solution remaining should be ensured as well.
- Secondary antibody causes. As with the primary antibody, check the secondary antibody and label concentration and incubation time. It is important to note that the secondary antibody chosen needs to be unbound to tissue immunoglobulins. Clean sections and no antibody solution remaining should be ensured as well.
- Chromogen causes. If the chromogen concentration is too high and the reaction time is too long, you need to reduce the chromogen concentration and shorten the incubation time. It is also possible that the counterstain is masking the IHC reaction and we suggest you replace a different counterstain. Clean sections and no excess stain remaining should be ensured as well.
We provide more information on IHC-Fr optimization protocols and troubleshooting to help you get the best results. Creative Biolabs also offers customized products and services to meet specific customer requirements, please contact us for scientific support.
Products with Tested Data
At Creative Biolabs, we are dedicated to providing high-quality antibodies for various research applications. Each product in our extensive range has been rigorously tested to ensure superior reliability and efficacy. To showcase the performance of our antibodies, we have conducted numerous experiments using Immunohistochemistry-Frozen (IHC-Fr). Below, you will find a table listing a selection of our antibody products along with images from these experiments, demonstrating their proven reliability.
| Product Name | Catalog Number | Target | Image | Description |
|---|---|---|---|---|
| Rabbit Anti-3-SLeX Recombinant Antibody (clone E2-B4) | VS9-YC2 | 3-SLeX |

|
The antibody was uesd for staining of the FFPE oesophageal adenocarcinoma tissue. The dilution range of 10-40 μg/mL is recommended. |
| Rabbit Anti-SSEA4 Recombinant Antibody (clone E2-F8) | VS9-YC1 | SSEA4 |

|
The antibody was uesd for staining of the FFPE oesophageal adenocarcinoma tissue. The dilution range of 10-40 μg/mL is recommended. |
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

