Enokizumab Overview
Introduction of Enokizumab
Enokizumab, also known as MEDI-528, is a humanized IgG1κ type monoclonal antibody direct against the cytokine interleukin-9 (IL-9). This drug has been investigated in the treatment for asthma. So far, enokizumab has been evaluated the safety, tolerance, pharmacokinetics and immunogenicity of subcutaneous injection. In addition, the safety, tolerance and efficacy of enokizumab in the treatment of mild persistent asthma in adults were studied. These tests mentioned above have been completed.
Mechanism of Action of Enokizumab
Interleukin-9 (IL-9), the target molecular of enokizumab, is a cytokine belonging to Interleukin-10 (IL-10) superfamily. IL-9 is mainly secreted by activated Th9 cells. In addition, mast cells, eosinophils, neutrophils, other lymphocyte subsets can also be secreted. IL-9 plays the corresponding biological effects by interacting with its ligand. After IL-9 binds to the corresponding receptor, tyrosine Tyr407 located on α chain of IL-9R is phosphorylated, resulting in the activation of JAK1, while another γc chain leads to the activation of JAK3. Further activation of signal transduction and transcription activators STAT1, STAT3 and STAT5, initiate a series of related gene expression. It is considered that the main effector cells of IL-9 include mast cells, T lymphocytes and antigen presenting cells. More experiments have shown that the immunobiological function of IL-9 is bidirectional, that is, it can not only promote the occurrence and development of some diseases, but also inhibit the development of some diseases. At present, it is believed that IL-9 is a pathogenic factor in hypersensitivity and autoimmune diseases, while in parasite infection and skin transplantation, IL-9 can promote the clearance of pathogens and the formation of graft immune tolerance. Studies have found that IL-9 plays an important role in the occurrence of a variety of diseases, such as autoimmune colitis, bronchial asthma, autoimmune encephalomyelitis and atopic dermatitis. In asthma, IL-9 is involved in airway inflammation and hyperreactivity. Enokizumab was designed as a monoclonal antibody targeting IL-9 for the treatment of asthma. After administration, enokizumab binds to IL-9, blocking the activity of IL-9 and preventing IL-9-mediated effects, to alleviate the symptoms of asthma.
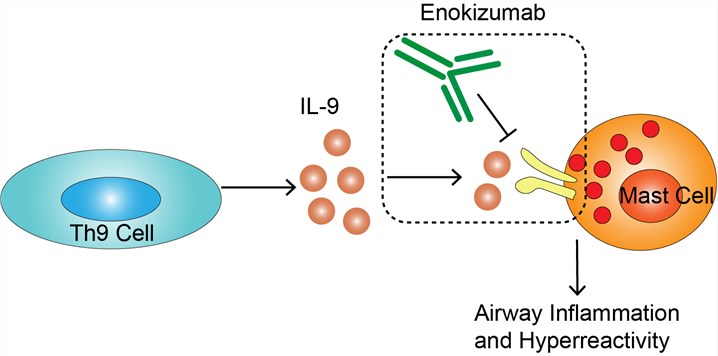 Fig.1 Mechanism of Action of Enokizumab
Fig.1 Mechanism of Action of Enokizumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



