Rovelizumab Overview
Introduction of Rovelizumab
Rovelizumab, also known as LeukArrest and Hu23F2G, is a recombinant humanized monoclonal antibody targeting at CD18. It was an experimental immunosuppressive drug developed by LCOS Corporation for the treatment of patients with hemorrhagic shock. Since 1997, rovelizumab has been explored in a variety of research projects, including phase II studies in MS patients experiencing acute exacerbations and a phase III trial for acute ischemic stroke. In addition, rovelizumab is also used in preclinical studies of cerebral vasospasm, head trauma, renal transplantation and restenosis. During testing, the number of patients in the trial was low because the drug needed to be administered within 4 hours after the injury, and the consent of the patient relatives needed to be reached as the patient himself was unconscious. In 2002, preclinical trials for cerebral vasospasm, CNS trauma, peripheral arterial occlusive disorders were discontinued, because the clinical data of the drug did not meet the expected standards.
Mechanism of Action of Rovelizumab
Rovelizumab is an IgG4 type monoclonal antibody, which can target CD18 to affect the migration and adhesion of target cells. CD18, also known as integrin beta-2, is a β subunit of integrin. It can be matched with four different forms of integrin α subunits to form different integrins. Among them, CD18 forms lymphocyte functional antigen-1 (LFA-1) in combination with CD11 α. LFA-1 is a member of leukocyte adhesion receptor and is expressed on all leukocytes, including B and T lymphocytes, monocytes, macrophages, neutrophils and basophils, but not in non-hematopoietic tissues and human platelets. By Binding to ICAMs, LFA-1 participates in the adhesion of leukocytes and lymphocytes to vascular endothelium and plays an important role in the local migration of leukocytes to inflammation and the homing and recirculation of lymphocytes. By binding to the integrin molecule containing CD18, rovelizumab prevents the binding of neutrophils to ICAMs and prevents the fine adhesion and migration of neutrophils.
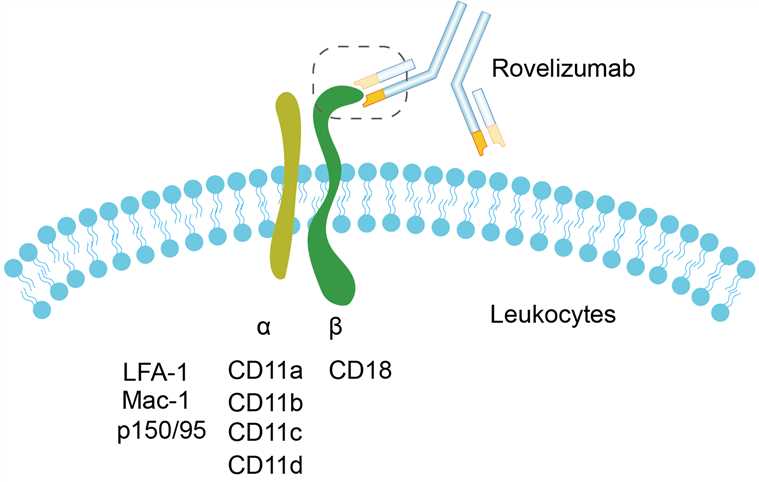 Fig.1 Mechanism of Action of Rovelizumab
Fig.1 Mechanism of Action of Rovelizumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

