Bimekizumab Overview
Introduction of Bimekizumab
Bimekizumab (formerly UCB4940) is a novel humanized monoclonal antibody of the immunoglobulin G1 isotype, rationally designed to be able to bind at a similar site on both proinflammatory cytokine interleukin (IL)-17A and IL-17F, conveying dual inhibition of both isoforms. It was discovered and developed for the treatment of psoriasis. Comparing this unique mechanism of action with other agents, bimekizumab might improve therapeutic efficacy through its potent selectivity for the IL-17A and IL-17F isoforms. UCB initiates enrolment in the phase-III BE RADIANT trial for plaque psoriasis in USA on July 13th, 2018.
Mechanism of Action of Bimekizumab
IL-17A can be produced by a range of different cell types as part of both the adaptive and innate immune responses, including neutrophils, macrophages, mast cells, Group 3 innate lymphoid cells, T helper 17 (Th17) cells and cytotoxic T cells. IL-17A is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. IL-17A plays a key role in the pathogenesis of plaque psoriasis, psoriatic arthritis and ankylosing spondylitis and is up-regulated in lesional skin in contrast to non-lesional skin of plaque psoriasis patients and in synovial tissue of psoriatic arthritis patients. The frequency of IL-17-producing cells was also significantly higher in the subchondral bone marrow of facet joints from patients with ankylosing spondylitis. Nevertheless, complete remission remains rare and many patients respond only partially, or not at all, to treatment blocking these cytokines. Therefore, novel dual-cytokine blockade may have a more profound impact on chronic tissue inflammation than targeting IL-17A alone. IL-17A shares greater than 50% structural homology and overlapping biological function with another IL-17 family member, IL-17F. Both cytokines are expressed by the same cell types; can be secreted as homodimers or IL-17A-IL-17F heterodimers; and signal through the same IL-17RA/RC receptor complex. IL-17A and IL-17F are both upregulated in a variety of inflamed human tissues and co-operate with other pro-inflammatory cytokines, such as tumour necrosis factor (TNF), to amplify inflammatory responses. Bimekizumab binds to and neutralizes IL-17A and IL-17F, preventing their interactions with the IL-17 receptors expressed on keratinocytes, fibroblast-like synoviocytes, endothelial cells, chondrocytes and osteoblasts. As a result, bimekizumab inhibits the release of proinflammatory cytokines, chemokines and mediators of tissue damage and reduces IL-17-mediated contributions to autoimmune and inflammatory diseases.
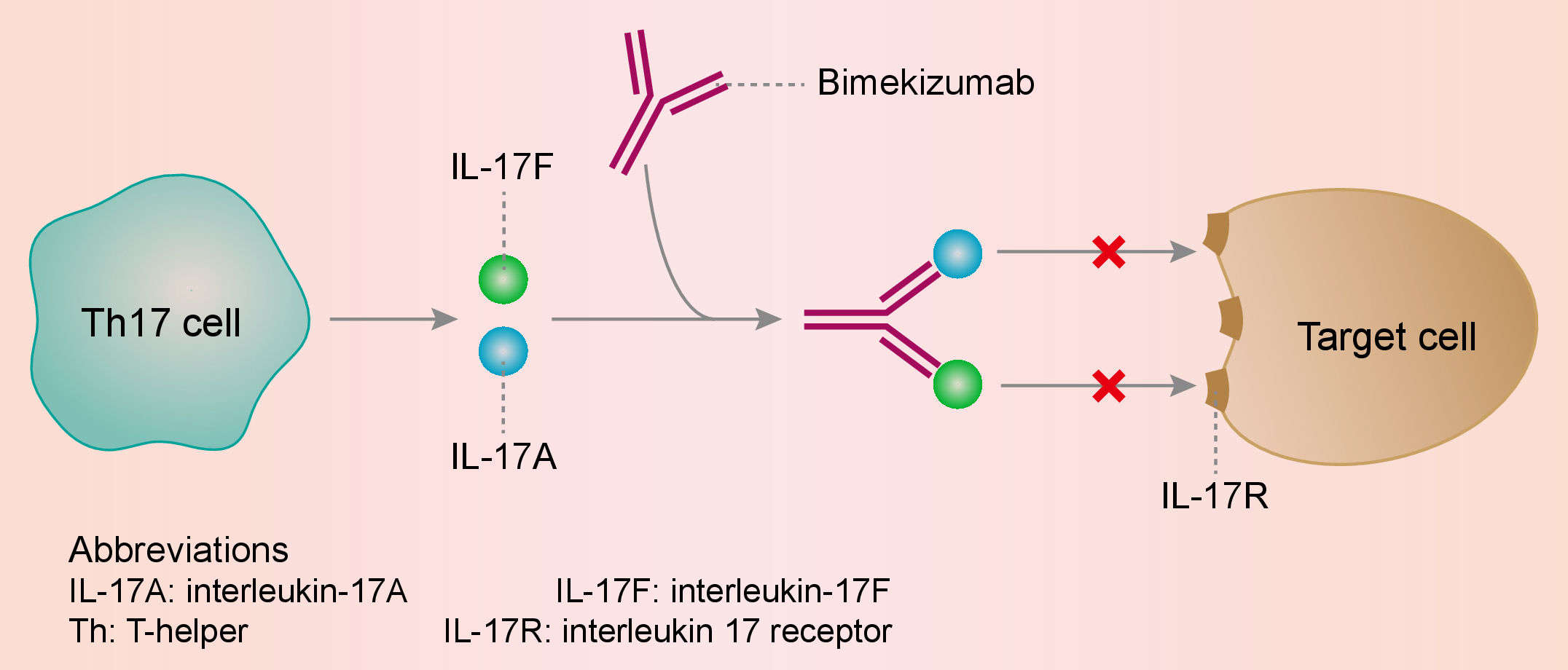 Fig.1 Mechanism of action of bimekizumab
Fig.1 Mechanism of action of bimekizumab
Clinical Projects of Bimekizumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03347110 | Enrolling by invitation | Psoriatic Arthritis | UCB Biopharma S.P.R.L. | November 20, 2017 |
| NCT03355573 | Enrolling by invitation | Ankylosing Spondylitis | UCB Biopharma S.P.R.L. | November 28, 2017 |
| NCT03536884 | Recruiting | Chronic Plaque Psoriasis, Moderate to Severe Chronic Plaque Psoriasis | UCB Biopharma S.P.R.L. | May 25, 2018 |
| NCT03412747 | Recruiting | Chronic Plaque Psoriasis, Moderate to Severe Chronic Plaque Psoriasis | UCB Biopharma S.P.R.L. | January 26, 2018 |
| NCT03230292 | Active, not recruiting | Chronic Plaque Psoriasis | UCB Biopharma S.P.R.L. | July 26, 2017 |
| NCT03370133 | Recruiting | Chronic Plaque Psoriasis, Moderate to Severe Chronic Plaque Psoriasis, Psoriatic Arthritis | UCB Biopharma S.P.R.L. | December 12, 2017 |
| NCT03010527 | Active, not recruiting | Chronic Plaque Psoriasis | UCB Biopharma S.P.R.L. | January 5, 2017 |
| NCT02963506 | Active, not recruiting | Ankylosing Spondylitis | UCB Biopharma S.P.R.L. | November 15, 2016 |
| NCT03410992 | Recruiting | Chronic Plaque Psoriasis, Moderate to Severe Chronic Plaque Psoriasis, Psoriatic Arthritis | UCB Biopharma S.P.R.L. | January 25, 2018 |
| NCT02969525 | Active, not recruiting | Psoriatic Arthritis | UCB Biopharma S.P.R.L. | November 21, 2016 |
| NCT03248531 | Active, not recruiting | Hidradenitis Suppurativa | UCB Biopharma S.P.R.L. | August 14, 2017 |
| NCT03215277 | Recruiting | Ankylosing Spondylitis | UCB Biopharma S.P.R.L. | July 12, 2017 |
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Bimekizumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

