Ofatumumab Overview
Introduction of Ofatumumab
Ofatumumab, also known as HuMax-CD20, is an IgG1ĸ type fully human monoclonal antibody directed against CD20 antigen that expressed on normal B lymphocytes (pre-B- to mature B-lymphocyte) and on B-cell CLL. The molecular weight of ofatumumab is approximately 149 kDa. This drug was designed for the treatment of chronic lymphocytic leukemia (CLL). Ofatumumab was also conditional approved by Health Canada on August 2012, and by European Medicines Agency on April 19, 2010 for the treatment of chronic lymphocytic leukemia. In addition, this drug also received Therapeutic Goods Administration (TGA) approval and Japan Medical Devices Evaluation Center approval in 2013. In addition to CLL, ofatumumab also has also shown potential in treating follicular lymphoma, diffuse large B cell lymphoma, rheumatoid arthritis and relapsing remitting multiple sclerosis.
Mechanism of Action Ofatumumab
Ofatumumab has been designed to attach to a protein called CD20. Ofatumumab was found to inhibit the early-stage B lymphocyte activation. Thus, Ofatumumab became the first human monoclonal antibody which targets the CD20 molecule that will be available for patients with refractory CLL. Ofatumumab was designed to bind specifically to both the small and large extracellular loops of the CD20 molecule. Upon administration, the Fab domain of ofatumumab binds to the CD20 molecule and the Fc domain mediates immune effector functions. Binding of ofatumumab to CD20 stimulates the body’s immune system to attack the cancerous B-cells, helping to control the disease. In addition, by attaching to CD20, ofatumumab also could induce recruitment and activation of the complement pathway at the cell surface, leading to complement-dependent cytotoxicity and resultant lysis of tumour cells, and induce cell death through antibody-dependent cell-mediated cytotoxicity.
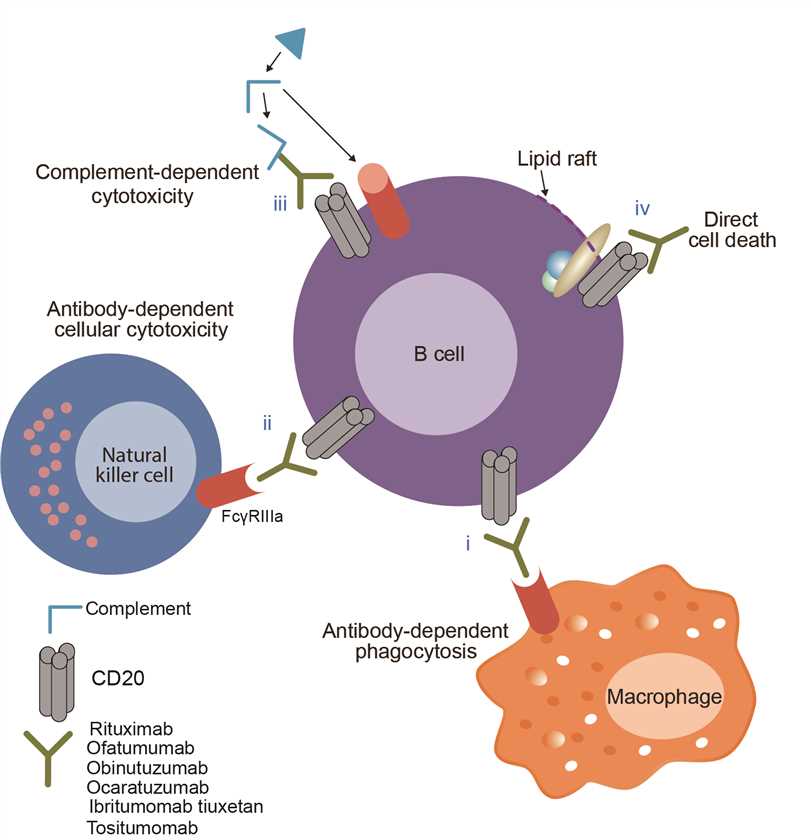
Fig.1 Mechanism of action of Ofatumumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



