Solanezumab Overview
Introduction of Solanezumab
Solanezumab is a humanized monoclonal IgG1 antibody being investigated as a neuroprotector or patients with Alzheimer's disease. It directed against the mid-domain of the soluble monomeric amyloid-β (Aβ). Solanezumab was safely used in combination with approved Alzheimer’s disease treatment, such as acetylcholinesterase inhibitors, in the clinical trials. Aside from Alzheimer’s disease, there are other amyloid beta related diseases, in which solanezumab can be used, e.g., down syndrome or cerebral amyloid angiopathy. However, this has not been studied so far.
Mechanism of Action of Solanezumab
The precise mechanism by which Solanezumab exerts its therapeutic effects in AD is unknown, but the therapeutic rationale is that it may exert benefits by sequestering Aβ, shifting equilibria between different species of Aβ, and removing small soluble species of Aβ that are directly toxic to synaptic function. In the preclinical research, a single injection of m266, the mouse version of solanezumab, reversed memory deficits in APP-transgenic mouse models while leaving amyloid plaques in place, raising the prospect of targeting the soluble pool of Aβ.
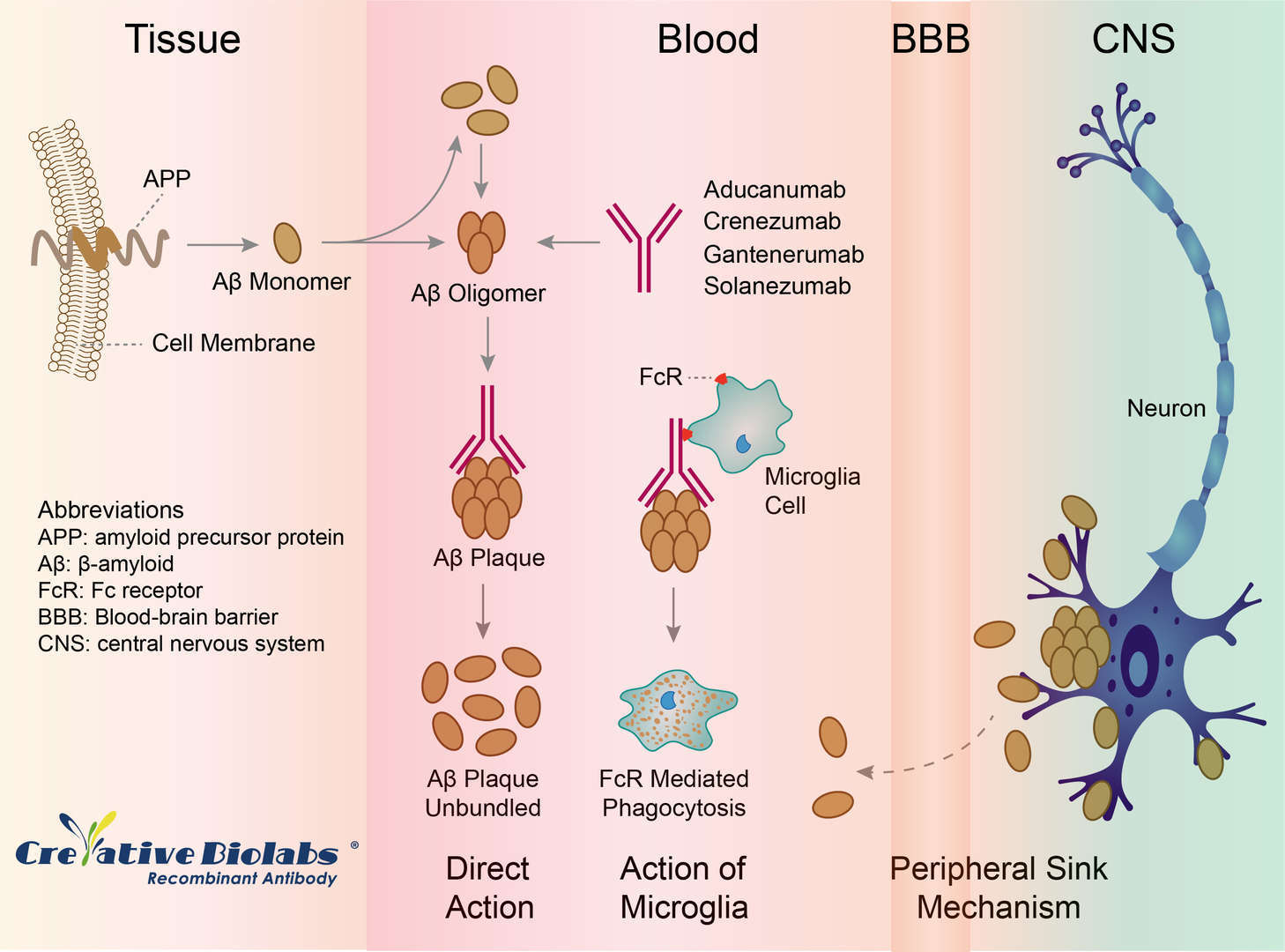 Fig.1 Mechanism of Action of Solanezumab
Fig.1 Mechanism of Action of Solanezumab
Clinical Projects of Solanezumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT02008357 | Active, not recruiting | Cognition Disorders | Eli Lilly and Company | December 11, 2013 |
Resource
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Solanezumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



