Lumiliximab Overview
Introduction of Lumiliximab
Lumiliximab is an IgG1κ type chimeric monoclonal antibody (mAb) that targets CD23. It acts as an immunomodulator and was awarded orphan drug status and fast track designation by the Food and Drug Administration (FDA). The drug is chimerized from Macaca irus and Homo sapiens antibodies. It has been investigated in clinical trials for the treatment of chronic lymphocytic leukemia (CLL), and been studied for use in allergic asthma (terminated in 2007). In preclinical studies, lumiliximab was shown to enhance the effects of fludarabine and rituximab, providing a rationale for combining lumiliximab with regimens containing fludarabine and rituximab in clinical trials in CLL. In a 46-patient, phase 1, dose-escalation trial performed in patients with relapsed and refractory CLL, lumiliximab monotherapy was well tolerated at doses of up to 500 mg/m2. Although no complete responses (CRs) s or partial responses (PRs) were noted in this trial. 52% patients had a decrease in lymph node bulk, and 91% patients had modest reduction in lymphocytosis. However, these effects were transient, with most patients progressing by 2 months after therapy. No additional benefit was observed with the more frequent dosing regimens at 500 mg/m2. In a phase 1/2 study of lumiliximab combined with fludarabine, cyclophosphamide and rituximab (FCR) in patients with relapsed or refractory CLL, results suggested that the addition of lumiliximab to FCR therapy is feasible, achieves a high CR rate, and does not appear to enhance toxicity in previously treated patients with CLL. However, in a phase 2/3 study to evaluate the safety and efficacy of lumiliximab in combination with FCR versus FCR alone in subjects with relapsed CLL, overall the combination of lumiliximab with FCR was not significantly better than FCR alone. There was a slightly increased incidence of adverse events with lumiliximab, but these increases did not appear to lead to differences in eventual outcomes. An interim analysis failed to show sufficient efficacy of the combination of lumiliximab with FCR. The study was therefore stopped early (in 2010) for lack of efficacy.
Mechanism of Action of Lumiliximab
The CD23 antigen (also termed FcRII), a type II transmembrane glycoprotein that is expressed on several hematopoietic cell types, functions as a low-affinity receptor for IgE. CD23 has been shown to play a role in modulating the production of IgE by B cells. The CD23 protein is a member of the C-type lectin family, and it contains an alpha-helical coiled-coil stalk between the extracellular lectin-binding domain and the transmembrane region that oligomerizes membrane-bound CD23 as a trimer. CD23 can undergo proteolytic cleavage in the stalk region, resulting in the release of soluble forms of CD23 from the cell surface. In addition to its involvement in regulating the production of IgE, it has been postulated that CD23 has various biologic functions, including promoting the survival of germinal center B cells. The expression of CD23 is highly up-regulated in normal activated follicular B cells and in CLL B cells. Lumiliximab is a primatized monoclonal anti-CD23 antibody that contains cynomolgus macaque variable regions and human constant regions (IgG1κ) that was developed to inhibit the production of IgE by activated human peripheral blood B cells. Lumiliximab does not mediate antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC) against CLL cells. Lumiliximab mediates caspase-dependent apoptosis through the mitochondrial death pathway and induces Bax activation and the release of proapoptotic mitochondrial proteins.
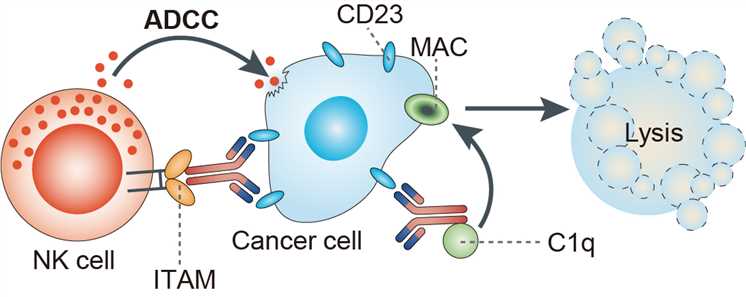
Fig.1 Mechanism of action of lumiliximab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



