Ibritumomab Tiuxetan Overview
Introduction of ibritumomab tiuxetan
Ibritumomab tiuxetan is an antibody drug that consists in a monoclonal mouse IgG1 antibody ibritumomab and a chelator tiuxetan. The radioactive isotope yttrium-90 or indium-111 is added to the chelator tiuxetan, which is a modified version of diethylenetriamine pentaacetic acid (DTPA) whose carbon backbone contains an isothiocyanatobenzyl and a methyl group. This drug is directed against CD20 antigen, which is found on the surface of normal and malignant B cells (but not B cell precursors). 2002, ibritumomab tiuxetan was approved by the Drug Administration (FDA) for the treatment of relapsed or refractory, low grade or transformed B cell non-Hodgkin's lymphoma. In 2004, ibritumomab tiuxetan was approved by European Medicines Agency and the U.S. Food for the treatment of follicular lymphoma.
Mechanism of Action of ibritumomab tiuxetan
The active substance in ibritumomab tiuxetan, ibritumomab, is a monoclonal antibody composed of two murine gamma 1 heavy chains of 445 amino acids each and two kappa light chains of 213 amino acids each and is designed to target an antigen, CD20. Radioactive element Indium or yttrium conjugated murine IgG1 kappa monoclonal antibody destroy the cell via production of beta particles when the Fab segment of the antibody binds to the CD20 epitope on B-cells. In addition, the antibody itself can cause cell death through antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and apoptosis. B cells are cleared from the body by the combination of antibodies and reflexive elements, but since there is no CD antigen in stem cells, healthy B cells can regenerate after treatment.
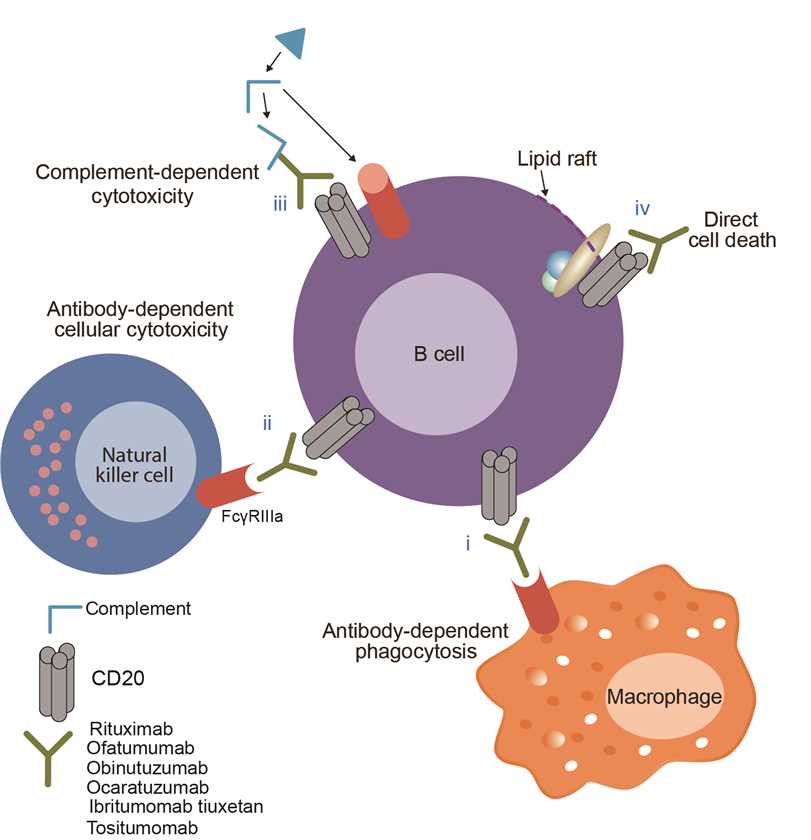
Fig.1 Mechanism of action of ibritumomab tiuxetan
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



