Burosumab Overview
Introduction of Burosumab
Burosumab (KRN23) is an investigational recombinant fully human monoclonal IgG1κ antibody against the phosphaturic hormone fibroblast growth factor 23 (FGF23) in development for X-Linked Hypophosphatemia (XLH). XLH is a bone disease characterized by phosphate-wasting due to excess activity of FGF23. FGF23 is a hormone that reduces serum levels of phosphorus and vitamin D by regulating phosphate excretion and vitamin D production by the kidney. Low phosphate levels lead to inadequate mineralization of bone, which leads to a spectrum of skeletal abnormalities, physical impairment, weakness, and pain. Most pediatric patients and some adult patients are managed using oral phosphate replacement and vitamin D (calcitriol) therapy, which requires frequent divided doses and careful medical monitoring. It is partially effective at reducing rickets in pediatric patients, but it does not improve growth and can be challenging to optimize the dose without increasing the risk of depositing phosphate-calcium precipitates in the kidneys (nephrocalcinosis). Burosumab was first approved in European Union on February 19, 2018. Subsequently it was approved by the Food and Drug Administration (FDA) for its intended purpose, in patients aged 1 year and older, on 17 April 2018. The FDA approval fell under both the breakthrough therapy and orphan drug designations. While burosumab is effective for the treatment of X-linked hypophosphatemia, the UK National Institute for Health and Care Excellence has raised concerns regarding the incremental cost-effectiveness of the new treatment.
Mechanism of Action of Burosumab
X-linked hypophosphatemia is caused by excess FGF23 which suppresses renal tubular phosphate reabsorption and the renal production of 1,25 dihydroxy vitamin D. Burosumab is designed to bind to and thereby inhibit the excessive biological activity of FGF23. By blocking excess FGF23 in patients with XLH, KRN23 is intended to restore normal phosphate reabsorption from the kidney and increase the production of vitamin D, which enhances intestinal absorption of phosphate and calcium.
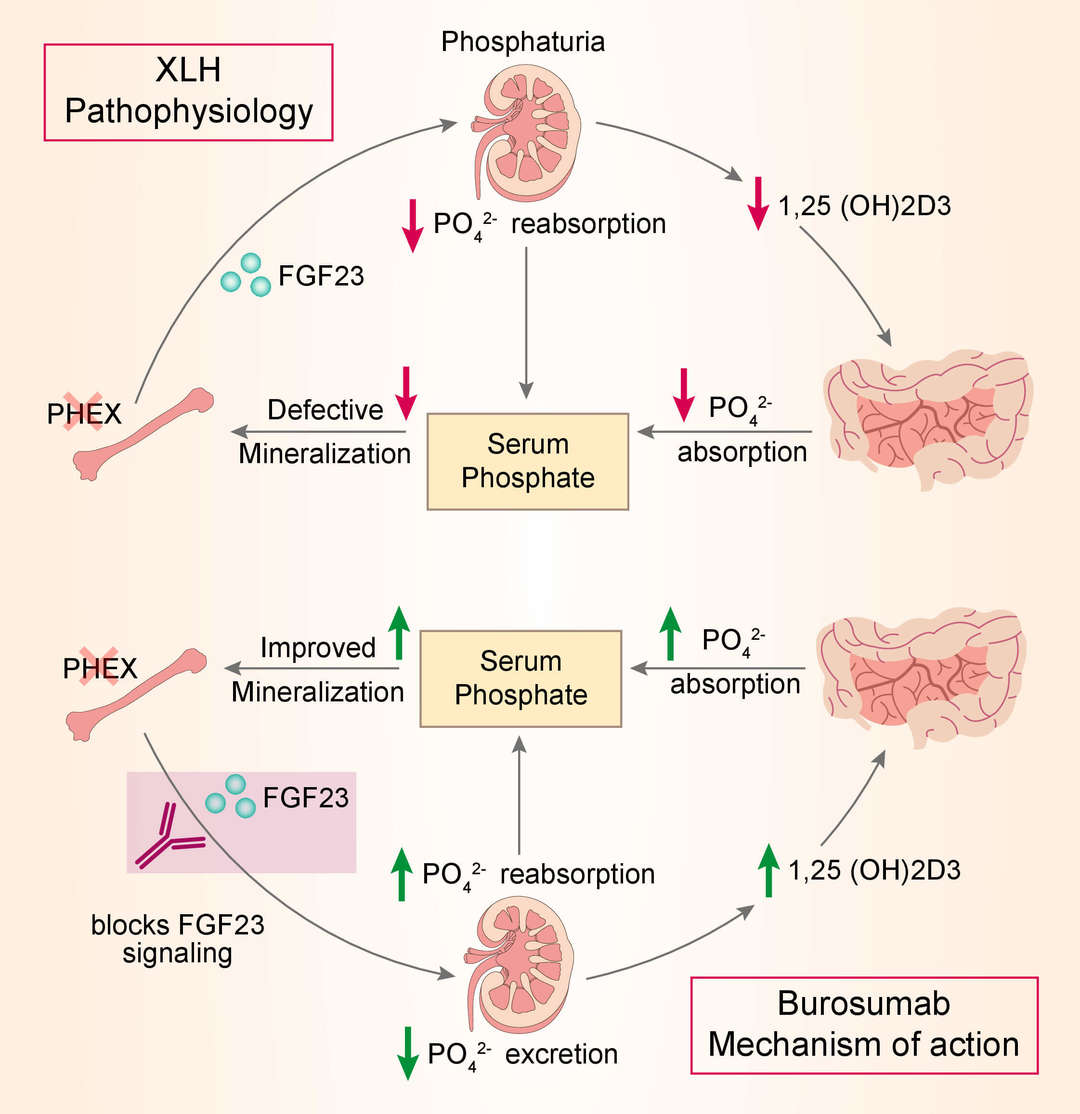 Fig.1 Mechanism of action of burosumab
Fig.1 Mechanism of action of burosumab
Clinical Projects of Burosumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03581591 | Recruiting | Hypophosphatemia, Hypophosphatemic Rickets, Chronic Pain | Redwood Dermatology Sciences | July 10, 2018 |
| NCT02526160 | Active, not recruiting | X-linked Hypophosphatemia | Ultragenyx Pharmaceutical Inc | August 18, 2015 |
Approved Drugs of Burosumab**
| INN (trade name) | Therapeutic area | Dose | Strength | Route | Company | Marketing start | Market |
| Crysvita | X-linked hypophosphatemia (XLH) | Solution |
10 mg/mL, 20 mg/mL, 30 mg/mL |
Subcutaneous | Ultragenyx Pharm Inc. | April 17, 2018 |

|
| Crysvita | Hypophosphatemia, Familial; Hypophosphatemic Rickets, X-Linked Dominant | Solution |
10 mg/mL, 20 mg/mL, 30 mg/mL |
Subcutaneous | Kyowa Kirin Limited | February 19, 2018 |

|
Resources
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Burosumab
** Information presented in the table were collected from the following websites:
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo= 761068
http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004275/human_med_002224.jsp
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

