Conatumumab Overview
Introduction of Conatumumab
Conatumumab (previously called AMG-655) is a fully human Immunoglobulin G1 (IgG1) type monoclonal binding to tumor necrosis factor receptor superfamily member 10B (TNFRSF10B) which could induce apoptosis in many types of human cancer cells. This drug was developed by Amgen Inc and Japanese licensee Takeda Bio Development Center Ltd with potential antineoplastic activity. This drug has been investigated in trials studying the treatment of sarcoma, lymphoma, oncology, colon cancer, and rectal cancer. Conatumumab can elicit the apoptosis in cell lines derived from colon and pancreatic cancers, as well as in mice bearing xenograft tumors in some in vitro and in vivo assays. Clinical trials in phase I has assessed the safety of conatumumab as a monotherapy as well as in combination with other antibody therapies or standard chemotherapeutic regimes. And the anti-conatumumab antibody responses have not been observed in previous trials. In addition, conatumumab has been found to enhance the antitumor activity of agents like irinotecan and gemcitabine in some preclinical researches. A trail about conatumumab combined with AMG 479 has shown that it is well-tolerated and no drug-drug interactions and the phase II development of this combination is ongoing.
Mechanism of Action of Conatumumab
Conatumumab is designed as a monoclonal agonist directed against TNFRSF10B, which is also known as death receptor 5 (DR5) and tumor necrosis factor related apoptosis-inducing ligand receptor 2 (TRAIL-R2). TNFRSF10B is mainly expressed by the immune system cells and belongs to the tumor necrosis factor family of cytokines. TNFRSF10B acts as a death receptor that transmits apoptotic signals through different signaling systems to induce apoptosis when stimulated by the external apoptotic initiator. Just like other members of TFN superfamily, the extracellular part of TNFRSF10B is a cysteine-rich region, and the intracellular region is a death domain with hydrolytic function, which is responsible for the trigger of intracellular apoptosis signal. When TNFRSF10B binds to its ligand, Fas-associated death domain (FADD) is recruited. FADD forms the death induced signal complex (DISC) with procaspase 8 through its N-terminal death effector domain (DED), stimulating and activating caspase-8 and caspase-10. After activation, caspase-8 catalyzes caspase protease to produce cascade amplification reaction, and finally plays the biological function of inducing cell apoptosis by activating its effector caspase-3. TNFRSF10B is found to be involved in signal pathways like gene expression and protein kinase B (Akt) signaling and is associated with several diseases such as squamous cell carcinoma, head and neck, and diffuse infiltrative lymphocytosis syndrome. TNFRSF10B can be detected on a variety of solid tumors and cancers of hematopoietic origin. In addition, it is more widely expressed on the surface of tumor cells than normal cells, and when it binds to related ligands, it can selectively kill a variety of tumor cells and is less toxic to normal cells. Thus, TNFRSF10B is considered as an ideal target molecule for developing anti-tumor specific monoclonal antibody drugs. The mechanism of action of conatumumab is to mimic the activity of the native TRAIL, binding to and activating TNFRSF10B, thereby activating caspase cascades. The activation of caspases leads to DNA fragmentation and tumor-cell apoptosis and death.
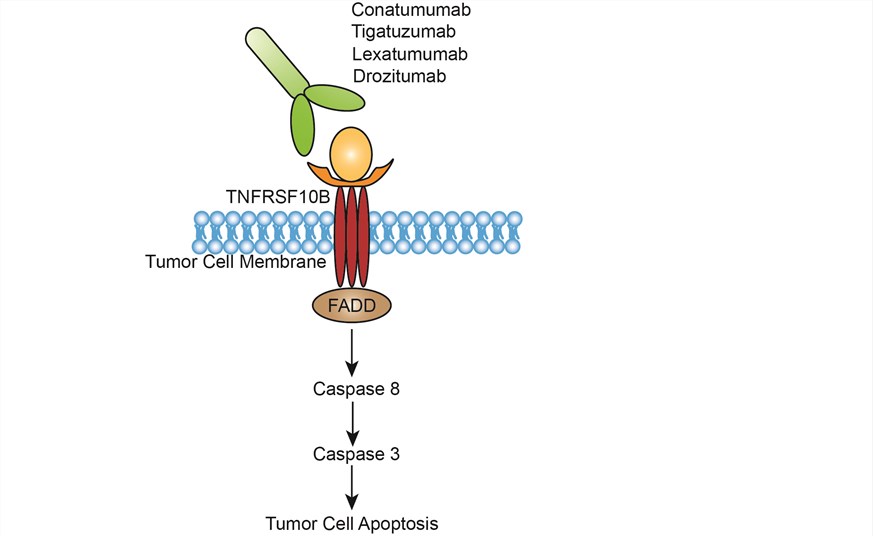 Fig.1 Mechanism of action of Conatumumab
Fig.1 Mechanism of action of Conatumumab
Table 1. Clinical Projects of Conatumumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT01327612 | Active, not recruiting | Advanced Solid Tumors, Carcinoid, Colorectal Cancer, Locally Advanced, Lymphoma, Metastatic Cancer, Non-Small Cell Lung Cancer, Sarcoma, Solid Tumors | Amgen | July 18, 2018 |
What We Provide
Therapeutic Antibody
Conatumumab
We provide high-quality Conatumumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
Reference
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?term=Conatumumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.


