Elezanumab Overview
Introduction of Elezanumab
Elezanumab, also under the development names AE12-1Y-QL and ABT-555, is a recombinant humanized IgG1 monoclonal antibody targeting the human repulsive guidance molecule A (RGMa, or RMGA). RGMa is a member of the repulsive guidance molecule family, implicated in various physiological and pathological processes. More specifically, RGMa is notorious for its role in inhibiting axonal growth and neural regeneration post-injury, contributing to the pathophysiology of various neurodegenerative conditions. Elezanumab was designed to bind with high affinity and specificity to RGMa, impeding its interaction with receptors such as Neogenin, thereby modulating its function. The development of elezanumab capitalizes on neutralizing RGMa's inhibitory actions to promote neural repair and augment recovery in diseases characterized by neural damage. The expanding landscape of clinical applications, particularly in spinal cord injury, stroke rehabilitation, multiple sclerosis, and traumatic brain injury, heralds the dawn of innovative therapeutic paradigms. Recently, The Food and Drug Administration has approved elezanumab to be an orphan drug as well as a Fast Track medication intended for the treatment of individuals who have suffered a spinal cord injury. As ongoing clinical trials unravel its full potential, elezanumab stands poised to revolutionize neuroregenerative medicine, offering renewed hope for individuals grappling with neural damage and degeneration.
Biological and Chemical Properties of RGMA
Protein Structure
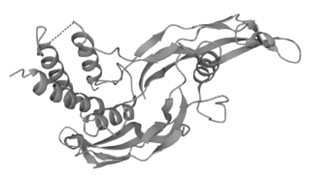 Figure 1. The Structure of Human RGMA (UniProt)1,2
Figure 1. The Structure of Human RGMA (UniProt)1,2
The Mechanism of Action of Elezanumab
The mechanism of elezanumab pivots around its antagonistic activity against RGMa, a glycosylphosphatidylinositol-anchored glycoprotein that exerts inhibitory effects on neuronal growth and regeneration. RGMa's physiological functions encompass the regulation of neural tube closure, axonal guidance, and neural regeneration. However, aberrant RGMa expression can impose detrimental effects in the context of neural injury and disease.
- Binding to RGMa: Elezanumab is engineered to specifically bind to RGMa, inhibiting its connection with Neogenin, a receptor pivotal to RGMa's inhibitory signaling pathways, as well as preventing its interaction with BMP-2 and BMP-4, which are known inhibitors of myelination in development and disease. By obstructing the binding of RGMa to other proteins, elezanumab impedes the downstream signaling cascade that culminates in growth cone collapse and inhibition of axonal outgrowth. This targeted approach results in an enhanced capacity for axonal regrowth and neural regeneration following injury.
- Modulation of Downstream Signaling: The RGMa-Neogenin interaction activates intracellular signaling pathways, notably the Rho/ROCK pathway, which orchestrates cytoskeletal dynamics crucial for axonal guidance and growth. Elezanumab's interference with RGMa binding to Neogenin attenuates the RhoA activation, subsequently suppressing the ROCK-dependent cytoskeletal rearrangements that inhibit axonal growth. This suppression fosters a cellular environment conducive to axonal sprouting, promoting repair and functional recovery in the damaged neural tissue. The Smad signaling pathway is another target of RGMa. Elezanumab potently inhibited RGMa-mediated BMP signaling via the SMAD1/5/8 pathway, preventing the phosphorylation and subsequent nuclear translocation of Smad proteins, thwarting their ability to regulate gene expression involved in axonal inhibition.
- Enhancement of Neuroplasticity: Beyond inhibiting RGMa-mediated growth cone collapse, elezanumab's action augments neuroplasticity. The enhanced regenerative capacity fosters synaptic reorganization and functional recovery, critical for restoring neural connectivity and performance post-injury or in degenerative conditions. Through its antagonistic effect on RGMa, elezanumab stimulates the intrinsic potential of neurons for regrowth and connectivity, thereby underpinning its therapeutic efficacy.
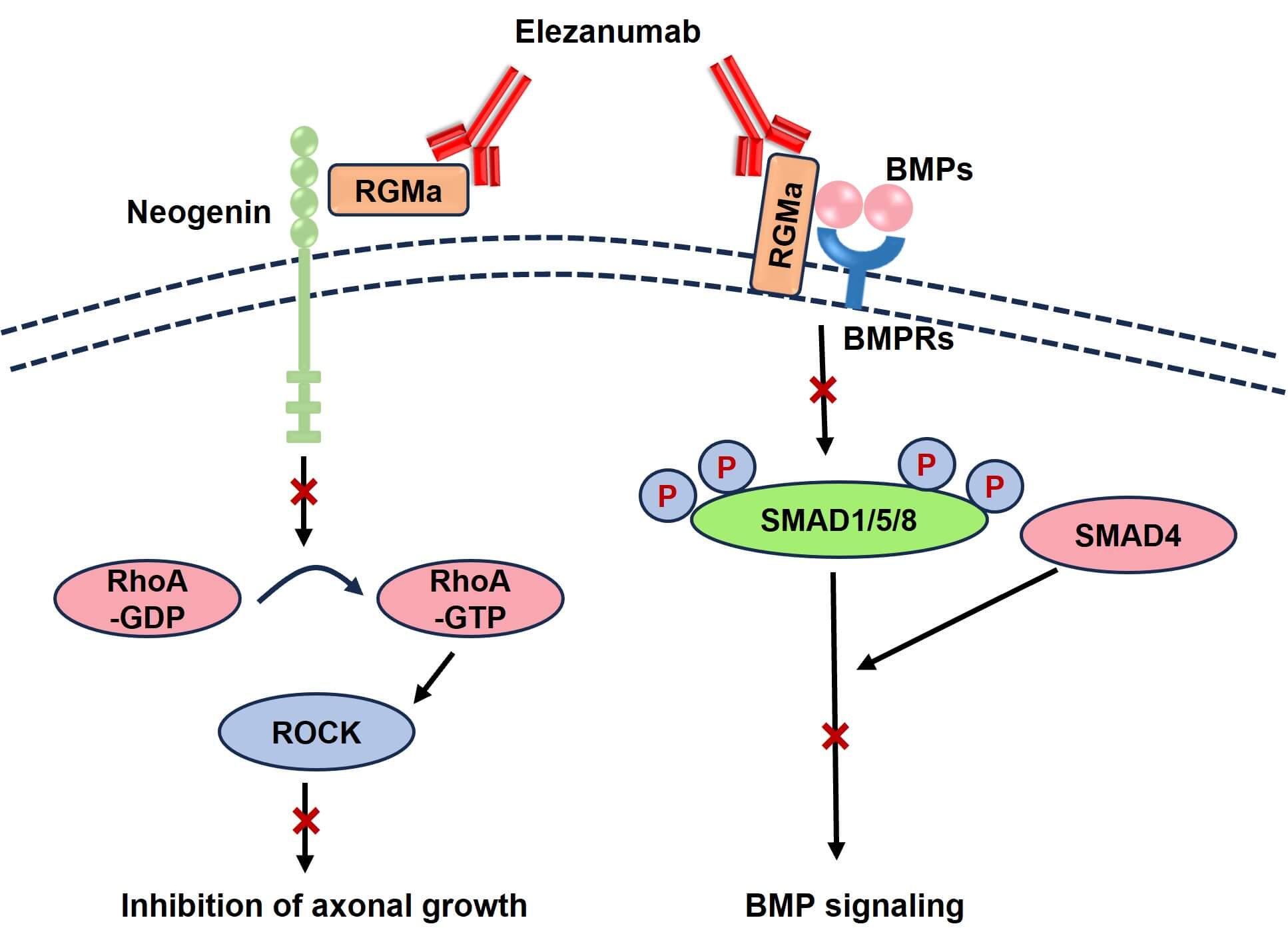 Figure 2. The Mechanism of Action of Elezanumab (Creative Biolabs Original)
Figure 2. The Mechanism of Action of Elezanumab (Creative Biolabs Original)
The Clinical Applications of Elezanumab
Elezanumab's potential spans a variety of clinical scenarios, particularly those involving neural injury and neurodegeneration. Preclinical and clinical investigations underscore its efficacy in addressing conditions where neural repair and regeneration are paramount.
- Spinal Cord Injury: Spinal cord injuries often culminate in irreversible damage due to inhibited axonal regeneration. Elezanumab's capability to neutralize RGMa emerges as a beacon of hope in ameliorating such injuries. Preclinical models exhibit enhanced axonal growth, functional recovery, and locomotor improvements upon elezanumab administration post-spinal cord injury. These promising results have propelled clinical trials to assess efficacy and safety in human subjects, aiming to translate preclinical success into tangible clinical outcomes.
- Stroke Rehabilitation: Stroke-induced neural damage and subsequent functional deficits pose formidable challenges in neurorehabilitation. Elezanumab's capacity to promote neural regeneration and synaptic plasticity harbors potential in stroke recovery. By facilitating axonal sprouting and synaptic reorganization, elezanumab may accelerate functional recovery and ameliorate neurological deficits post-stroke. Ongoing clinical trials are exploring its utility in augmenting stroke rehabilitation protocols, with initial findings suggesting improved outcomes in motor and cognitive functions.
- Multiple Sclerosis (MS): In multiple sclerosis, demyelination and axonal damage contribute to progressive neurological decline. Elezanumab's antagonism of RGMa offers a novel therapeutic avenue in MS by promoting remyelination and axonal repair. Preclinical studies demonstrate enhanced axonal regrowth and functional recovery in animal models of MS, underscoring its relevance in managing this debilitating condition. Clinical trials are underway to evaluate its safety and efficacy in individuals with MS, seeking to halt or reverse disease progression.
- Traumatic Brain Injury (TBI): Traumatic brain injury results in complex neural damage, often culminating in cognitive and motor impairments. Elezanumab's role in promoting neural repair and plasticity positions it as a potential therapeutic agent in TBI management. Preclinical investigations have depicted improved cognitive outcomes and neural repair post-TBI with elezanumab treatment. These promising findings warrant further clinical exploration to establish its efficacy in enhancing recovery trajectories following TBI.
Clinical Projects of Elezanumab*
| NCT ID | Study Title | Study Status | Conditions | Sponsor | Start Date |
| NCT03737812 | A Study to Assess the Safety and Efficacy of Elezanumab When Added to Standard of Care in Progressive Forms of Multiple Sclerosis | Completed | Multiple Sclerosis (MS) | AbbVie | 2019-02-27 |
| NCT03737851 | A Study to Assess the Safety and Efficacy of Elezanumab When Added to Standard of Care in Relapsing Forms of Multiple Sclerosis | Completed | Multiple Sclerosis (MS) | AbbVie | 2018-12-11 |
| NCT04309474 | A Safety and Efficacy Study of Intravenous (IV) Elezanumab Assessing Change in Neurologic Function in Adult Participants With Acute Ischemic Stroke | Active, not recruiting | Acute Ischemic Stroke | AbbVie | 2020-11-09 |
* The table was excerpted from the following website: https://clinicaltrials.gov/search?cond=Elezanumab
- Uniprot Database (https://www.uniprot.org/uniprotkb/Q96B86/entry#function)
- The image was retrieved from UniProt Database and used under [CC BY 4.0]. It was not modified and the titles was "The Structure of Human RGMA (UniProt)".
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

