Elsilimomab Overview
Introduction of Elsilimomab
Elsilimomab is a murine monoclonal antibody (mAb), also known as B-E8, which has been the subject of several proof-of-concept clinical studies in hematological malignancies diseases. IUnpublished in vitro studies showed that mAb 1339 shares similar biological properties, including affinity and epitope specificity, with its “parent” murine antibody elsilimomab (B-E8). Humanized mAb 1339 has shown in vitro and in vivo anti-multiple myeloma activity, as well as inhibition of bone turnover, providing a rationale for its clinical evaluation in multiple myeloma (MM).
Mechanism of Action of Elsilimomab
Multiple myeloma (MM) is caused by the proliferation of malignant plasma cells. Although the pathogenesis of the disease still remains unclear, research in the biology of MM has produced insights into the factors that control the growth and survival of myeloma cells. Among the growth factors, IL-6 has an essential role. Plasma cells themselves may produce autocrine IL-6 while IL-6 production by bone marrow stromal cells may operate a paracrine mechanism. Involvement of IL-6 in multiple myeloma is indicated by its ability to induce the differentiation of myeloma plasma blasts into mature malignant plasma cells. Differential diagnosis between multiple myeloma and monoclonal gammopathies of undetermined significance (MGUS) is generally based on clinical and laboratory parameters. Nevertheless, evaluation of the serum level of IL-6, C reactive protein, soluble IL-6 receptor, soluble IL-2 receptor together with the activity exerted by IL-3 and IL-4 on some cellular subsets constitutes an additional element in the differential diagnosis of border-line cases. Serum levels of IL-6, soluble IL-6 receptor (sIL-6R), soluble interleukin-2 receptor (sIL-2R) and the expression of membrane-bound IL-2 receptors, both on bone marrow plasma cells and on peripheral blood mononuclear cells are correlated with disease activity and disease stage. In addition, IL-6 and sIL-6R serum levels correlate with the duration of survival, as high values at the time of diagnosis correlate with short duration of survival. Based on these findings, elsilimomab (B-E8) and mAb 1339 were designed to target IL-6 and inhibit the interaction between IL-6 and IL-6R for the treatment of MM.
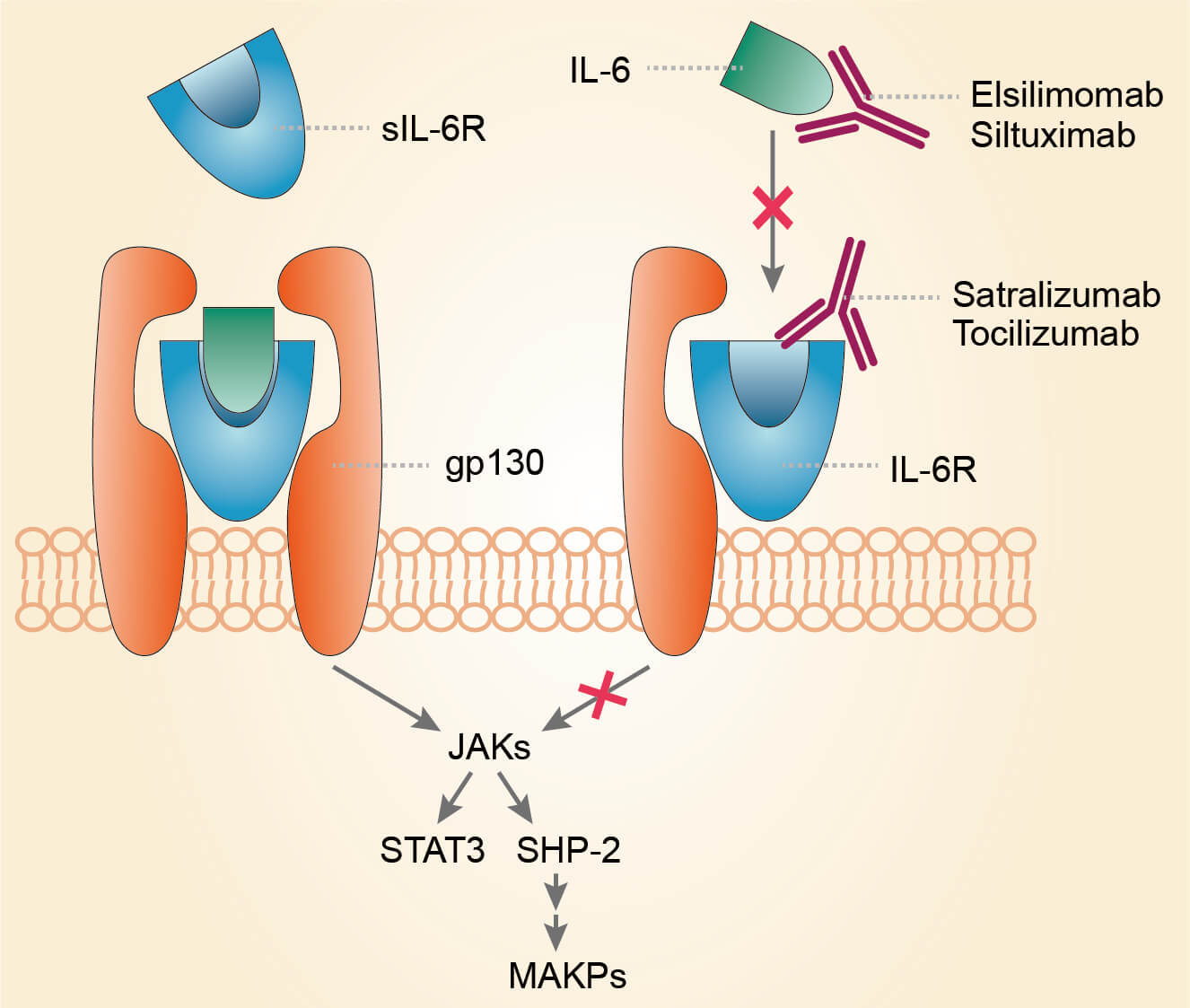 Fig.1 Mechanism of action of elsilimomab
Fig.1 Mechanism of action of elsilimomab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

