Enfortumab Vedotin Overview
Introduction of Enfortumab Vedotin
Enfortumab vedotin is an antibody-drug conjugate (ADC) designed for the treatment of cancer expressing Nectin-4. It was developed through two main lines, hybridoma (ASG-22ME) and Chinese hamster ovary (ASG-22CE). Enfortumab refers to the fully humanized (from mouse) monoclonal antibody (mAb), it is the first agent to target Nectin-4 that expressed on many solid tumors especially on bladder cancers. Vedotin refers to the payload drug microtubule-disrupting agent monomethyl auristatin E (MMAE) and the linker. Preclinical studies showed that enfortumab vedotin effectively binds to target cells, internalizes and induces cell-killing activity. Mouse and patient xenograft models were used to test enfortumab vedotin’s antitumor activity in human breast, bladder, pancreatic and lung cancers.
Mechanism of Action of Enfortumab Vedotin
Nectin and nectin-like proteins form a family of adhesion molecules that belong to the immunoglobulin superfamily. They play a key role in different biological processes such as cell polarity, proliferation, differentiation and migration in epithelial, endothelial, immune and nervous systems. Besides their role in physiology, they serve as virus receptors (poliovirus and herpes simplex virus) and are involved in orofacial malformation and play roles in cancer pathology. Among them, necl-5, nectin-2 and nectin-4 are overexpressed in tumors, and are associated with a poor prognosis. On the opposite, necl-1, necl-2 and necl-4 act as tumor suppressors and are repressed in cancer. Nectin-4, also known as poliovirus receptor-related protein 4 (PVRL4), is a type I transmembrane protein. Copy number gain of the PVRL4 gene is a frequent event in carcinogenesis and promotes epithelial-to-mesenchymal transition, invasion and metastasis through integrin-, PI3K/Akt- and Wnt/β-catenin-signaling pathways. While nectin-4 protein is expressed in several tumors, it is particularly prevalent in breast cancer and urothelial cancer. Nectin-4 expression in normal tissue is limited, which renders it a valuable target in epithelial cancers. As an ADC, enfortumab vedotin consists of a human anti-nectin-4 antibody conjugated to the anti-mitotic agent MMAE, it binds to nectin-4 on the surface of cancer cells, followed by internalization of the conjugate by endocytosis and release of its cytotoxic payload MMAE after lysosomal degradation. MMAE is a synthetic antineoplastic agent. Because of its toxicity, it cannot be used as a drug itself; instead, it is usually linked to a mAb which directs it to the cancer cells. MMAE disrupts microtubules and induces apoptosis of the tumor cell. In addition to the direct cytotoxic effect of MMAE at the cellular level, the antitumor effect of enfortumab vedotin can be mediated through additional mechanisms involving signal transduction inhibition from direct binding, antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).
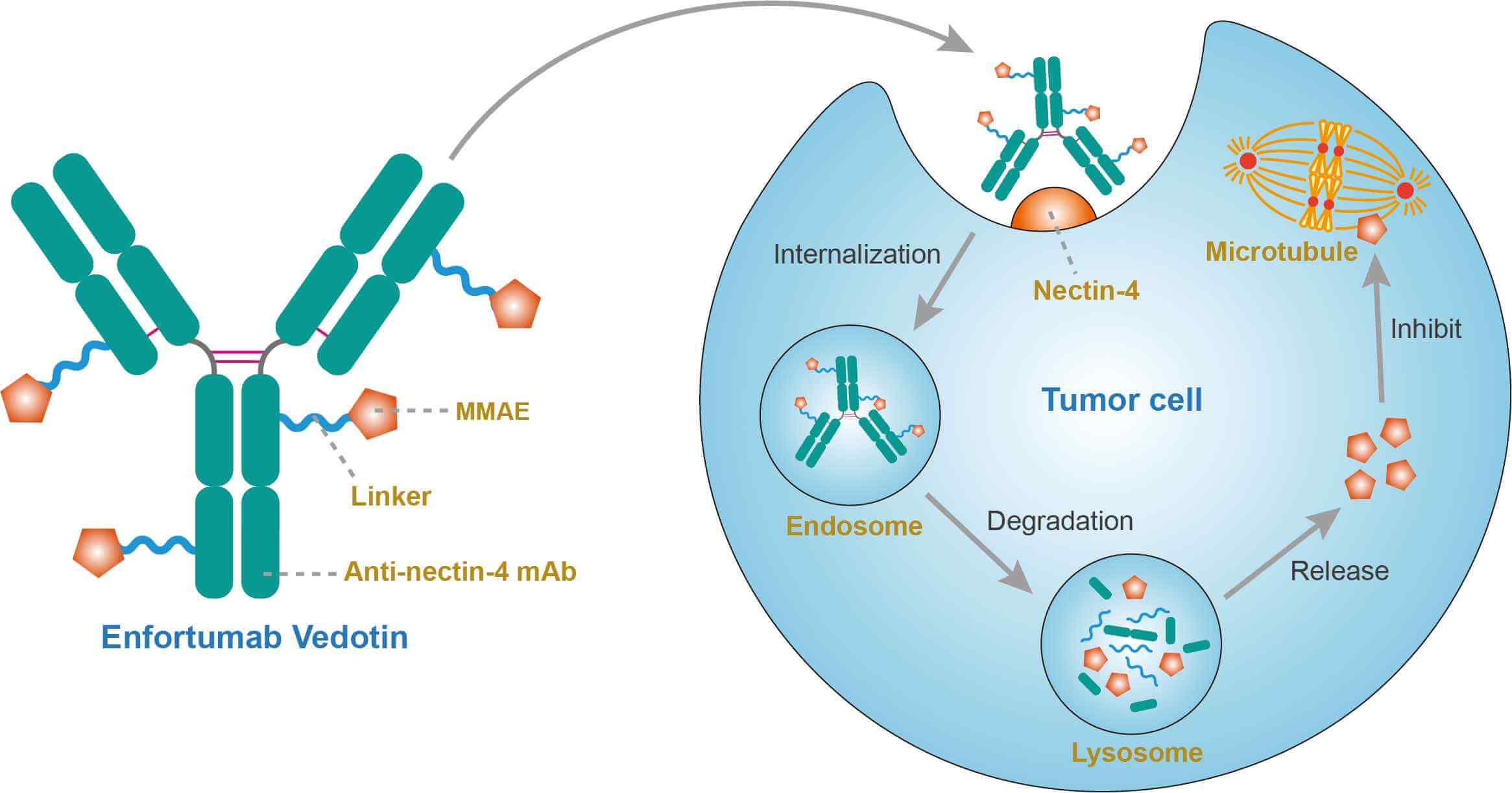 Fig.1 Mechanism of action of enfortumab vedotin
Fig.1 Mechanism of action of enfortumab vedotin
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

