Faralimomab Overview
Introduction of Faralimomab and IFNA1
Faralimomab is an investigational therapeutic antibody that targets interferon alpha-1 (IFNA1) and has gained considerable interest because of its capacity to modulate immune activity in autoimmune and inflammatory diseases. By acting on IFNA1, it provides a novel, directed way to control the immune system – and may be used in everything from systemic lupus erythematosus to rheumatoid arthritis and beyond. More research is needed before we can know its clinical utility, but Faralimomab's mechanism of action makes the case for its future as an immunological revolution.
IFNA1 and Disease: A Key Role for Autoimmunity and Chronic Inflammation
IFNA1, one of the types I interferons, regulates immune defense in response to viral infections. However, its overexpression or dysregulated signaling can be harmful, particularly in autoimmune conditions. IFNA1 is normally secreted by immune cells to counter infection and, in general, activates innate immunity by increasing the antiviral capabilities of immune cells such as dendritic cells, macrophages, and natural killer (NK) cells. It also activates signaling pathways that facilitate the production of antiviral proteins and pro-inflammatory cytokines, leading to a strong immune response.
But in autoimmune conditions, like systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and other disorders in which the immune system is overstimulated, IFNA1 fuels inflammation. When its expression in high levels, immune cells keep getting activated and release inflammatory mediators, provoking autoimmune reactions. In SLE, for instance, high IFNA1 is correlated with disease activity, such as skin rashes, joint pain, and kidney damage. This unnatural stimulation of IFNA1 causes it to enhance the production of other inflammatory cytokines, perpetuating a cycle of immune dysregulation that can damage tissues throughout the body.
Biological and Chemical Properties of IFNA1
Protein Structure
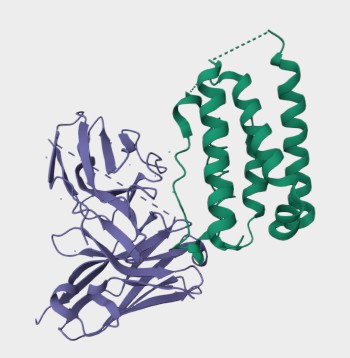 Figure 1. The Structure of Human IFNA1 (UniProt)1,2.
Figure 1. The Structure of Human IFNA1 (UniProt)1,2.
The Mechanism of Faralimomab Action: Neutralizing IFNA1
The therapeutic advantage of Faralimomab is that it suppresses the biological function of IFNA1. Overproduction of IFNA1 triggers a cascade of immune responses in autoimmune conditions, which in turn destroy tissue. Faralimomab works by blocking the signaling pathway, most notably the JAK-STAT pathway, which drives the expression of pro-inflammatory genes. By blocking IFNA1, Faralimomab reduces the production of immune cells, such as T cells and dendritic cells, that fuel autoimmune responses. This mechanism has made Faralimomab a promising candidate for the treatment of diseases with a high degree of type I interferon activity, including SLE and other chronic inflammatory diseases.
In SLE, an autoimmune chronic disease, the immune system attacks the body's tissues. One of the main pathological hallmarks of SLE is elevated levels of type I interferons such as IFNA1. These high levels of interferon fuel autoreactive immune cells and pro-inflammatory cytokines, catalyzing the inflammatory cascade and causing tissue damage. Faralimomab's capability to silence IFNA1 may relieve these symptoms by suppressing the over-activation of the immune system that underlies SLE. Research has demonstrated that the inhibition of type I interferons in animal models of lupus could dramatically mitigate disease progression and save organs. By targeting IFNA1 directly, Faralimomab provides a less invasive and potentially less toxic therapeutic approach than conventional immune-suppressants.
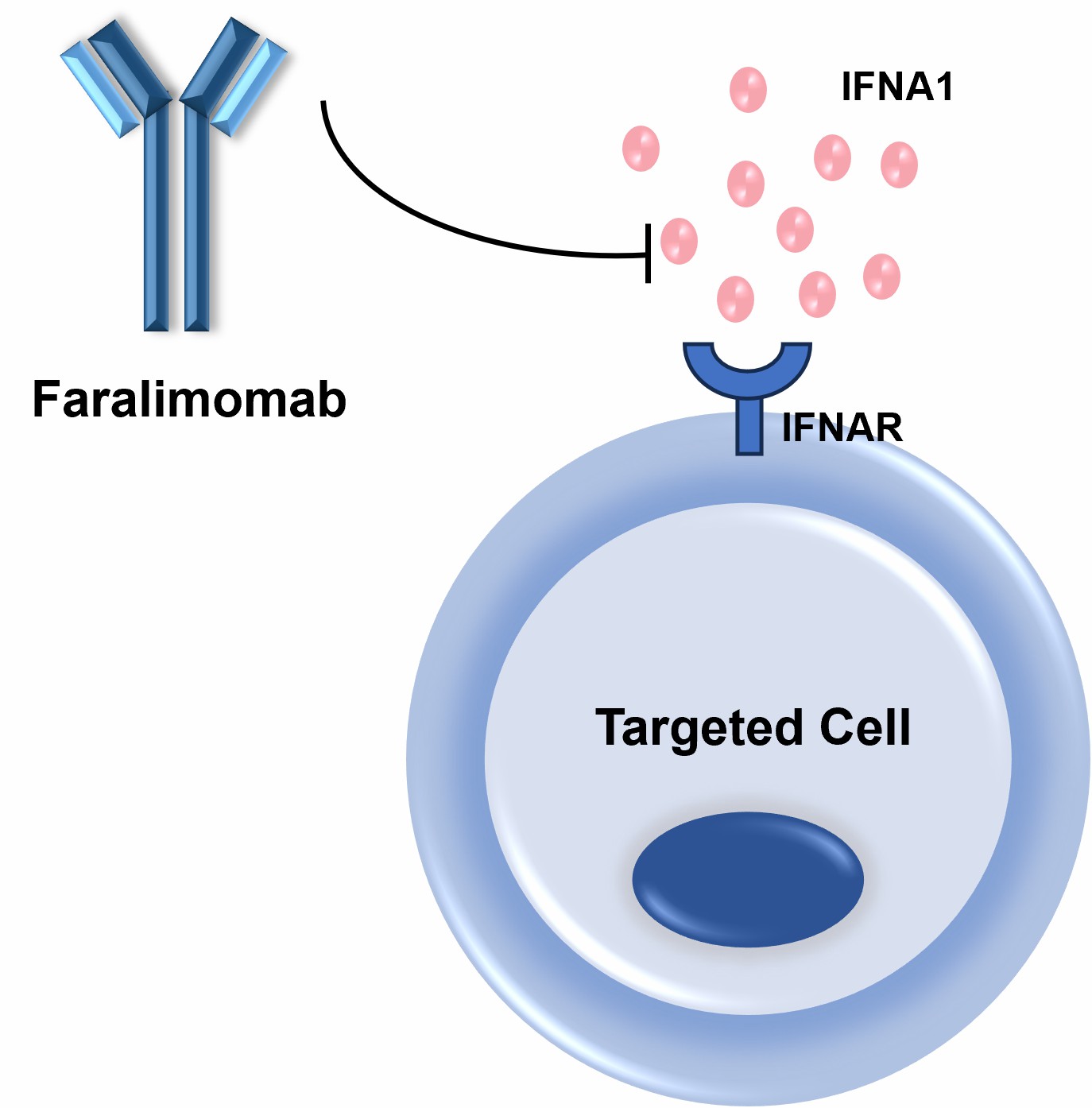 Figure 2. The Mechanism of Faralimomab Action.
Figure 2. The Mechanism of Faralimomab Action.
The Clinical Applications of Faralimomab
- Systemic Lupus Erythematosus (SLE): Faralimomab inhibits IFNA1, reducing immune activation and potentially controlling disease flare-ups in SLE patients.
- Rheumatoid Arthritis (RA): By neutralizing IFNA1, Faralimomab may reduce joint inflammation and slow disease progression in RA.
- Psoriasis: Faralimomab may help reduce skin inflammation and keratinocyte proliferation by targeting IFNA1.
- Inflammatory Bowel Disease (IBD): By neutralizing IFNA1, Faralimomab may alleviate intestinal inflammation and reduce flare-ups in IBD patients.
- Asthma: Targeting IFNA1 could reduce airway inflammation and improve asthma symptoms.
- Cancer Immunotherapy: Faralimomab may enhance the efficacy of cancer immunotherapies by modulating IFNA1 signaling in the tumor microenvironment.
What We Provide
Anti-IFNA1 Recombinant Antibody (Faralimomab)
We provide high-quality faralimomab for use in FuncS, IF, Neut, ELISA, FC, IP, IHC and most other immunological methods. The product is for lab research use only, not for diagnostic, therapeutic, or any in vivo human use.
- Immunogen
- The details of the immunogen for this antibody are not available.
- Host Species
- Mouse
- Derivation
- Mouse
- Type
- IgG1
- Species Reactivity
- Human
- Applications
- Suitable for use in FuncS, IF, Neut, ELISA, FC, IP, IHC and most other immunological methods.
- CAS
- 167816-91-3
- Generic Name
- faralimomab
- Related Disease
- Rheumatoid arthritis (RA)
- UniProt Database (https://www.uniprot.org/uniprotkb/P01562/entry#function)
- The image was retrieved from UniProt Database and used under [CC BY 4.0] without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



