Frunevetmab Overview
Introduction of Frunevetmab
Frunevetmab, or Solensia, is a feline monoclonal antibody that targets nerve growth factor (NGF). NGF contributes to pain and inflammation, especially in osteoarthritis (OA). Osteoarthritis is a degenerative joint disease characterized by the progressive breakdown of joint cartilage and subsequent inflammation. In cats, OA is more prevalent than commonly thought, with research indicating that 61% to 93% of cats show radiographic evidence of degenerative joint disease, and about 40% exhibit clinical signs of pain and mobility impairment. OA pain in cats can result in decreased activity, jumping aversion, and changes in grooming and behavior. Cats suffering from OA pain have always been under-treated. Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used but are not always safe for long-term use in cats and may cause side effects, particularly in those with concurrent conditions such as chronic kidney disease.
In 2022, the U.S. Food and Drug Administration designated frunevetmab as the first monoclonal antibody approved for use in animals. The biologic is indicated to treat OA-associated pain in cats and serves as a gap filler for cat pain. The most powerful pharmaceutical company, Zoetis, found its safety and efficacy in placebo-controlled trials. Frunevetmab has revolutionized pain management in veterinary medicine and represents a new possibility for extending the lifespan of elderly cats with degenerative joint disease.
Biological and Chemical Properties of NGF
Protein Structure
 Figure 1. The Structure of NGF (Wikipedia)1,2.
Figure 1. The Structure of NGF (Wikipedia)1,2.
The Mechanism of Frunevetmab Action
Frunevetmab's efficacy stems from its inhibition of nerve growth factor (NGF), an essential player in the pain signaling pathway. NGF is a neurotrophic factor involved in the sensitization of nociceptors (pain-sensing nerve fibers). It is released in response to inflammation and tissue injury, binding to receptors like tropomyosin receptor kinase A (TrkA) and p75 neurotrophin receptor (p75NTR) on nerve endings. This interaction amplifies pain signaling and contributes to chronic pain states such as OA. By binding to NGF, frunevetmab sequesters it and prevents its interaction with these receptors. This blockade interrupts the sensitization of nociceptors and mitigates the transmission of pain signals to the brain, effectively reducing pain perception in cats.
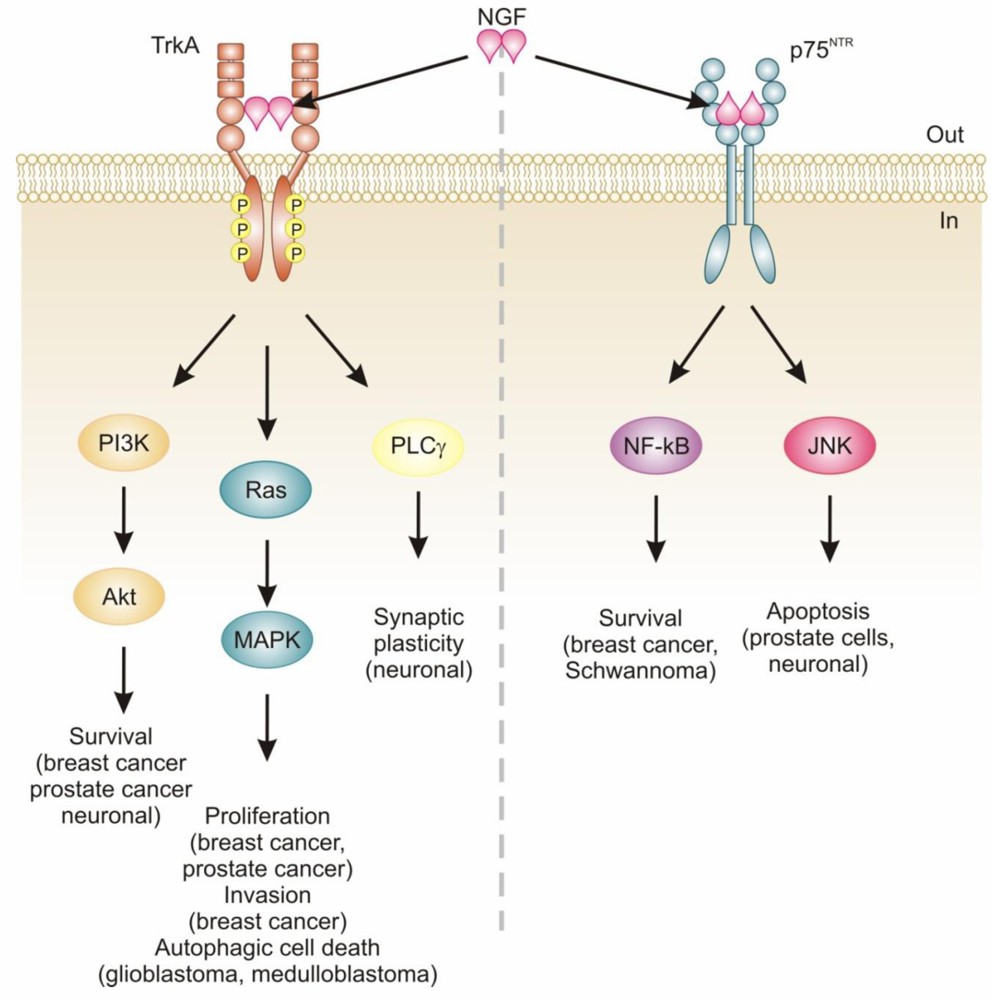 Figure 2. NGF/TrkA/p75NTR Signaling3.
Figure 2. NGF/TrkA/p75NTR Signaling3.
Clinical Trials for Frunevetmab
Frunevetmab's efficacy and safety have been demonstrated in a series of randomized, placebo-controlled studies of more than 400 client-owned cats with osteoarthritis (OA). In one trial, nearly three-quarters of cats on frunevetmab showed clinically significant reductions in pain and mobility impairment by day 56, compared to six-tenths in the placebo group. Also, 75.91% of cats treated with frunevetmab achieved treatment success on the Client-Specific Outcome Measures (CSOM) score, compared with 64.65% of cats in the placebo group. As for safety, side effects were mild and rare, mostly related to the skin, including injection site reactions (observed in 17.6% of treated cats) and transient dermatitis. No significant systemic adverse events directly related to the therapy were identified, confirming its long-term safety. The trials also showed long-term efficacy, with pain relief and mobility improvements observed after repeated monthly administrations. These findings establish frunevetmab as a novel and powerful treatment for feline OA, significantly improving cats' quality of life.
- Wikipedia (https://commons.wikimedia.org/wiki/File:NGF_protein.png)
- The image was retrieved from Wikipedia and used under [CC BY-SA 4.0] without modification.
- Molloy, Niamh H., Danielle E. Read, and Adrienne M. Gorman. "Nerve growth factor in cancer cell death and survival." Cancers 3.1 (2011): 510-530. Distributed under Open Access license CC BY 3.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

