Futuximab Overview
Introduction of Futuximab
Futuximab is a new drug that targets the epidermal growth factor receptor (EGFR), a protein involved in cell proliferation and repair. Overactivity of EGFR can lead to the growth of cancers such as colorectal cancer and squamous cell carcinoma of the head and neck (SCCHN). By attaching to the extracellular region of EGFR, futuximab causes the receptor to internalize and dissolve, stopping cancer cells from growing and reproducing. What makes futuximab unique is its ability to target not only normal EGFR but also mutant EGFR forms, such as EGFRvIII, which are resistant to most therapies. With its double targeting, it is promising in patients with resistant or aggressive cancers. Today, futuximab is being trialed in a number of countries and territories, including a Phase III study on solid tumors. The National Medical Products Administration (NMPA) in China has officially accepted its application for a Phase III clinical trial as a potential new treatment for patients with metastatic colorectal cancer. The first-in-class dual-antibody therapy (futuximab/modotuximab)–sym004, which binds independent, non-overlapping epitopes to EGFR, represents novel treatment targets for the epidermal growth factor receptor (EGFR). The combination of these approaches improves receptor internalization and degradation for better antitumor activity.
Biological and Chemical Properties of EGFR
Protein Structure
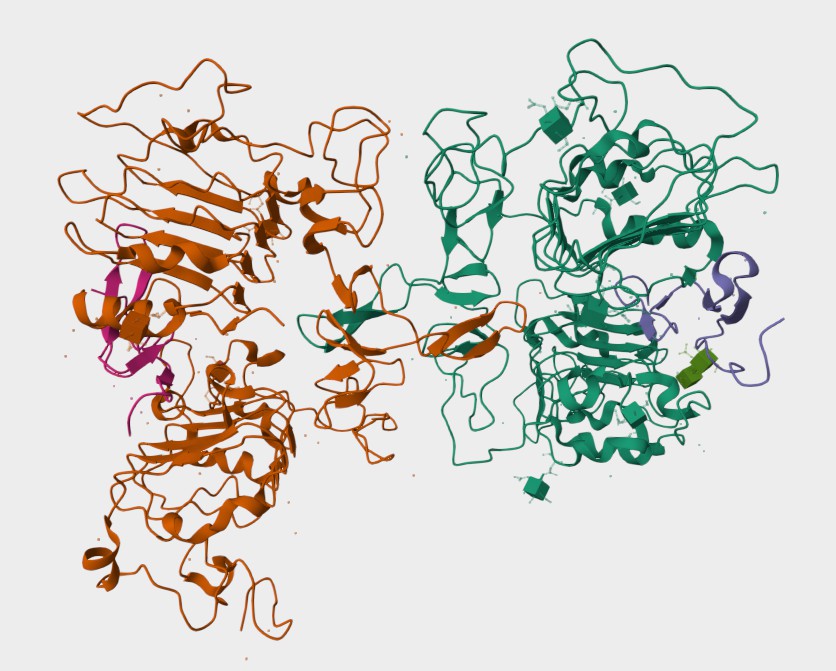 Figure 1. The Structure of Human EGFR (UniProt)1,2.
Figure 1. The Structure of Human EGFR (UniProt)1,2.
The Mechanism of Futuximab Action
EGFR as a Therapeutic Target
The epidermal growth factor receptor (EGFR) is like a cellular control switch that helps regulate key processes such as cell growth, division, and survival. This protein, as a part of the ErbB family of receptors, sits on the surface of cells and gets activated when certain molecules, like epidermal growth factor (EGF) or transforming growth factor-alpha (TGF-α), bind to it. Once activated, EGFR sends signals down specific pathways inside the cell, such as RAS-RAF-MEK-ERK and PI3K-AKT-mTOR, to ensure everything functions smoothly. However, in cancer, EGFR can go haywire. In many tumors, it is overproduced or mutated, leading to constant activation—even in the absence of external signals. This uncontrolled signaling drives cancer cells to grow uncontrollably, spread to other parts of the body, and create their own blood supply to feed the tumor. A common mutation, EGFRvIII, remains perpetually "on" without requiring activation signals. This makes it resistant to many standard treatments that aim to block EGFR, contributing to tumor progression and making cancers even harder to treat.
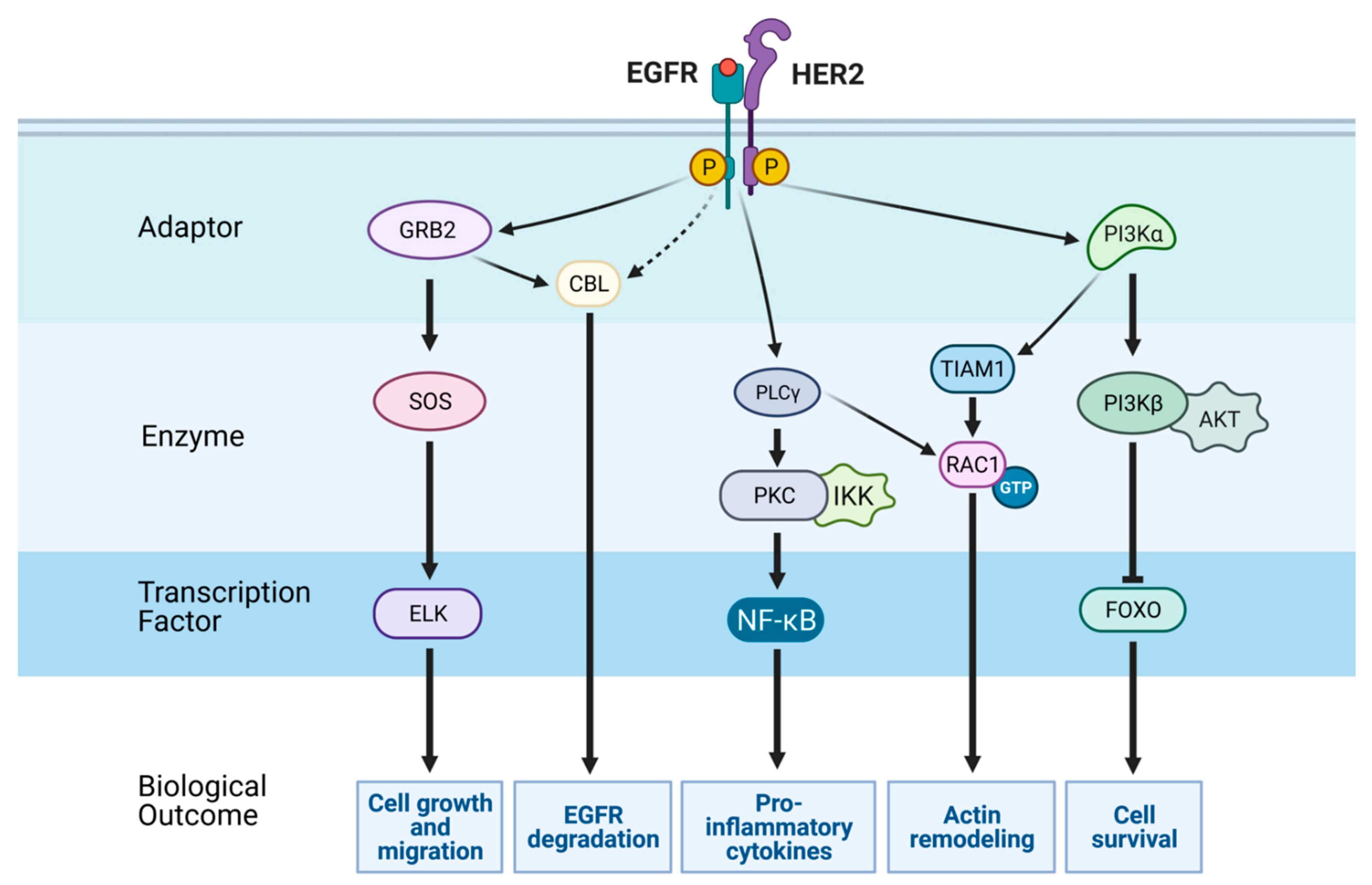 Figure 2. EGFR-mediated Signaling Pathways3,5.
Figure 2. EGFR-mediated Signaling Pathways3,5.
How Does Futuximab Work?
Futuximab works by locking onto the epidermal growth factor receptor (EGFR), a protein on the surface of cells that plays a crucial role in their growth and division. In healthy cells, EGFR activity is tightly controlled, but in many cancers, it becomes overactive, fueling tumor growth. Futuximab is designed to block this process by attaching to EGFR's extracellular domain, preventing it from interacting with its natural partners like epidermal growth factor (EGF). By doing so, it stops the activation of signals that instruct cancer cells to grow and multiply. What makes futuximab particularly powerful is its ability to target both normal EGFR and mutant forms like EGFRvIII, which are notoriously tough to treat because they're always "switched on" in some cancers, such as glioblastomas.
But futuximab doesn't just block EGFR—it takes things a step further by helping the body break it down. Once it binds to EGFR, it triggers a process that pulls the receptor inside the cell, directing it to the cellular "recycling center," where it is destroyed. This reduces the number of receptors on the cell surface, further weakening the cancer cell's ability to survive. At the same time, futuximab activates the immune system to join the fight against cancer. It recruits natural killer cells and other immune defenders to attack and destroy the cancer cells, adding another layer of defense. This multifaceted mechanism—disrupting growth signals, degrading EGFR, and activating the immune system—makes futuximab an incredibly effective weapon against cancers that rely on EGFR for survival.
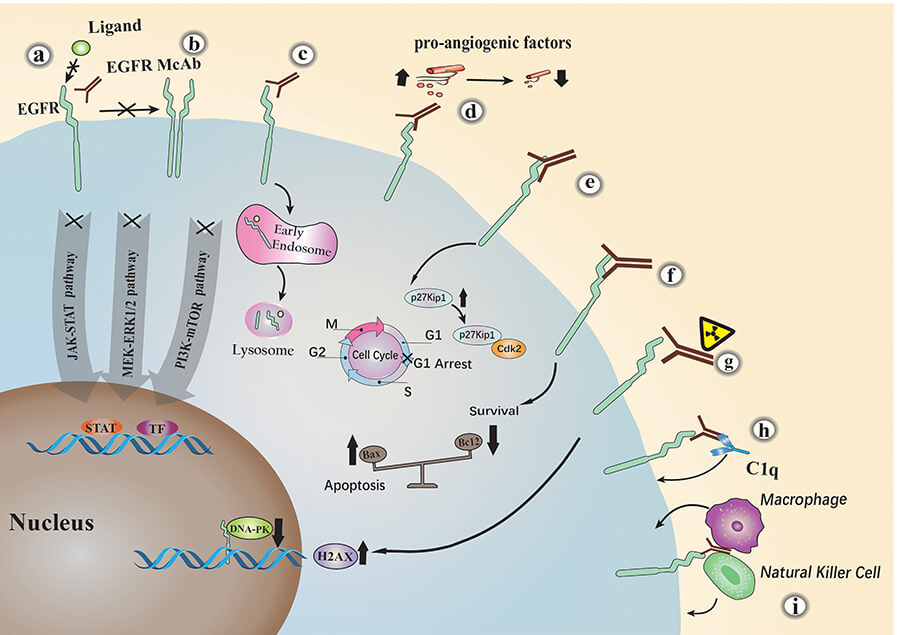 Figure 3. The Major Anti-tumor Mechanisms of EGFR Monoclonal Antibodies4,5.
Figure 3. The Major Anti-tumor Mechanisms of EGFR Monoclonal Antibodies4,5.
The Clinical Applications of Futuximab
- Metastatic Colorectal Cancer (mCRC): In colorectal cancer, EGFR is often overactive, driving tumor growth and spread. Futuximab works well in patients with certain genetic profiles (wild-type KRAS/NRAS and BRAF) who have already tried standard treatments without success. By targeting EGFR, futuximab helps slow down or stop tumor growth, offering hope for those who have few options left.
- Squamous Cell Carcinoma of the Head and Neck (SCCHN): This type of cancer often has high levels of EGFR, making it hard to control. Futuximab, especially when used as part of the Sym004 combination (futuximab plus modotuximab), has been effective in patients with advanced SCCHN who didn't respond to other therapies. Clinical studies have shown that this approach can shrink tumors or at least stabilize the disease, improving patients' quality of life.
- Glioblastoma (GBM): This aggressive brain cancer is notoriously hard to treat, partly because of mutant forms of EGFR like EGFRvIII, which keep signaling tumor cells to grow. Futuximab has shown promise in targeting and breaking down these mutated receptors, offering a potential new treatment avenue for glioblastoma patients.
Clinical Projects of Sym004*
| NCT ID | Study Title | Study Status | Conditions | Sponsor | Start Date |
| NCT01117428 | Sym004 in Patients With Advanced Solid Tumors | Completed | Metastatic Colorectal Cancer | Symphogen A/S | 2010-05-05 |
| NCT01955473 | Japanese Phase 1 Trial of Sym004 in Solid Tumors | Completed | Solid Tumors | Merck KGaA, Darmstadt, Germany | 2013-10-07 |
| NCT02540161 | Phase 2 Study of Sym004 for Adult Patients With Recurrent Glioblastoma | Completed | Malignant Glioma | Annick Desjardins | 2015-09-03 |
| NCT01417936 | Sym004 in SCCHN Patients Failing Anti-EGFR Based Therapy | Completed | Carcinoma, Squamous Cell of Head and Neck | Symphogen A/S | 2011-08-16 |
* The table was excerpted from the following website: https://clinicaltrials.gov/search?cond=Sym004&page=1
- UniProt Database (https://www.uniprot.org/uniprotkb/P00533/entry)
- The image was retrieved from UniProt Database and used under [CC BY 4.0] without modification.
- Uribe, Mary Luz, Ilaria Marrocco, and Yosef Yarden. "EGFR in cancer: Signaling mechanisms, drugs, and acquired resistance." Cancers 13.11 (2021): 2748.
- Cai, Wen-Qi, et al. "The latest battles between EGFR monoclonal antibodies and resistant tumor cells." Frontiers in oncology 10 (2020): 1249.
- Distributed under Open Access license CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



