Gilvetmab Overview
Introduction of Gilvetmab
Gilvetmab is a caninized monoclonal antibody based on blocking programmed cell death protein 1 (PDCD1 or PD-1), a key immune checkpoint in dogs. PD-1 is also found in activated T cells and regulates immunity by limiting the immune system's overactivation. However, cancer cells routinely take advantage of this to sneak past immune detection by expressing PD-L1 and PD-L2, ligands that bind to PD-1 and kill T cells. Gilvetmab knocks out this mechanism of action and breaks down the "brakes" on the immune system. It gives T cells a chance to attack cancer cells again. Gilvetmab inhibits PD-1 and by targeting the body's immune system, it offers a targeted, immune-mediated solution to cancers including mast cell tumors (MCTs) and melanoma, common in dog oncology. Creative Biolabs, a pioneer in antibody engineering, has created gilvetmab with the latest in recombinant technologies to make it highly specific and immunogenic.
The Mechanism of Gilvetmab Action
PD-1 is also a regulator of immune tolerance that keeps T cells from wrongly attacking normal tissue. PD-L1/PD-L2 is also upregulated by cancer cells in the tumor microenvironment, binding to PD-1 and turning off T cells. Such immunosuppression allows tumors to multiply unrestrained. Gilvetmab interrupts this process by targeting PD-1 directly. PD-L1 has been identified in more than half of dog mast cell tumors and melanomas, and it is a promising target for treatment. Gilvetmab, which silences PD-1, restores T-cell function and also activates other immune cells, including natural killer (NK) cells, to amplify the antitumor effect.
This mechanism proves especially helpful in advanced cancers where surgery or chemotherapy are ineffective. In dogs with metastatic melanoma, where the prognosis is typically poor, immune checkpoint inhibitors such as gilvetmab provide a new means of disease control.
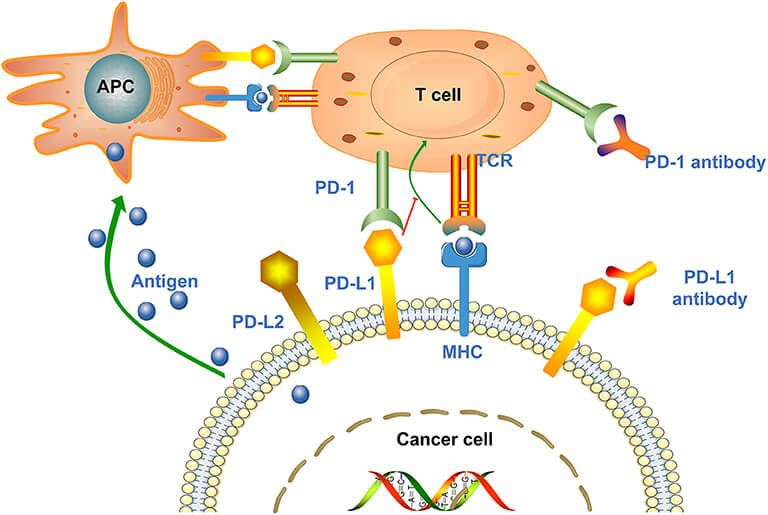 Figure 1. The Mechanism of Action of Anti-PD1 Abs (Gilvetmab) and Anti-PDL1 Abs1.
Figure 1. The Mechanism of Action of Anti-PD1 Abs (Gilvetmab) and Anti-PDL1 Abs1.
The Clinical Applications of Gilvetmab
Mast Cell Tumors (MCTs)
Mast cell tumors account for approximately 20% of canine skin cancers. These tumors are notoriously unpredictable, ranging from benign to highly aggressive forms. In clinical trials, gilvetmab demonstrated significant efficacy against MCTs. Over a 20-week treatment period, 73% of dogs showed either tumor shrinkage or disease stabilization. This remarkable response highlights its potential as a first-line therapy, particularly for dogs with inoperable or recurrent tumors.
Data from the trial also revealed a median progression-free survival (PFS) of 16 weeks, a significant improvement compared to historical controls treated with conventional therapies. Additionally, the durable response observed in many patients underscores the sustained immune activation achieved through PD-1 inhibition.
Melanoma
Canine melanoma, accounting for 7% of malignant tumors, is aggressive and often resistant to standard treatments. Gilvetmab has shown promise in this context, with 60% of treated dogs experiencing a reduction in tumor size or stable disease. In one subset of dogs with advanced metastatic melanoma, median overall survival (OS) was extended by 24 weeks, demonstrating the antibody's ability to improve outcomes in late-stage cancer.
These results position gilvetmab as a transformative option in the management of canine cancers, particularly for cases where conventional treatments have limited efficacy.
- Su, Chaoyue, et al. "Adverse effects of anti-PD-1/PD-L1 therapy in non-small cell lung cancer." Frontiers in oncology 10 (2020): 554313. Distributed under Open Access license CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

