Navivumab Overview
Introduction of Navivumab
Navivumab (Ct-P23) is a very specific monoclonal antibody specifically designed for the inhibition of IAV influenza. Its activity relies on its binding to the HA protein, a surface glycoprotein of vital importance on the IAV virion. In particular, Navivumab interferes with the stem region of HA's HA2 subunit shared by different IAV subtypes. By binding to this highly conserved domain, Navivumab blocks the conformational alterations required for viral entry, thereby providing a treatment that is effective against a large variety of IAV strains.
CT-P27 from Celltrion is a combination of two monoclonal antibodies, CT-P22 and CT-P23, which will respond to all Influenza A subtypes, including H1N1, H3N2, and H5N1. This helps to prevent the virus from escaping from the immune system by binding to segments of the virus's hemagglutinin protein. CT-P27 has been highly effective in preclinical studies, and it has already passed phase I trials testing safety and biochemistry. Currently in phase IIa, it's being tested on an H3N2 challenge system and is promising with oseltamivir in combination.
The Mechanism of Action of Navivumab
Navivumab works by targeting the HA2 stem region of the hemagglutinin (HA) protein, which is essential for the virus to enter host cells. The HA protein facilitates the fusion of the viral envelope with the host cell membrane, a critical step in infection. When the virus enters the cell, the HA protein undergoes a pH-induced change inside the endosome, exposing a fusion peptide that helps the virus merge with the host cell. By binding to the HA2 stem, Navivumab blocks this crucial fusion process, preventing the virus from releasing its genetic material and halting the infection early.
What makes Navivumab particularly effective is its ability to neutralize a broad range of Influenza A subtypes. This is possible because it targets conserved regions of the HA protein—areas that are less likely to mutate compared to other parts of the protein. These conserved regions are vital for the virus's ability to fuse with host cells, making them a stable target for long-term protection against evolving strains.
In addition to its direct antiviral action, Navivumab also has the potential to boost the body's immune response. The Fc region of the antibody can interact with immune cells like macrophages and dendritic cells, enhancing their activity. This could lead to a stronger immune defense, helping the body clear infected cells and recruit additional immune factors to fight the virus more effectively.
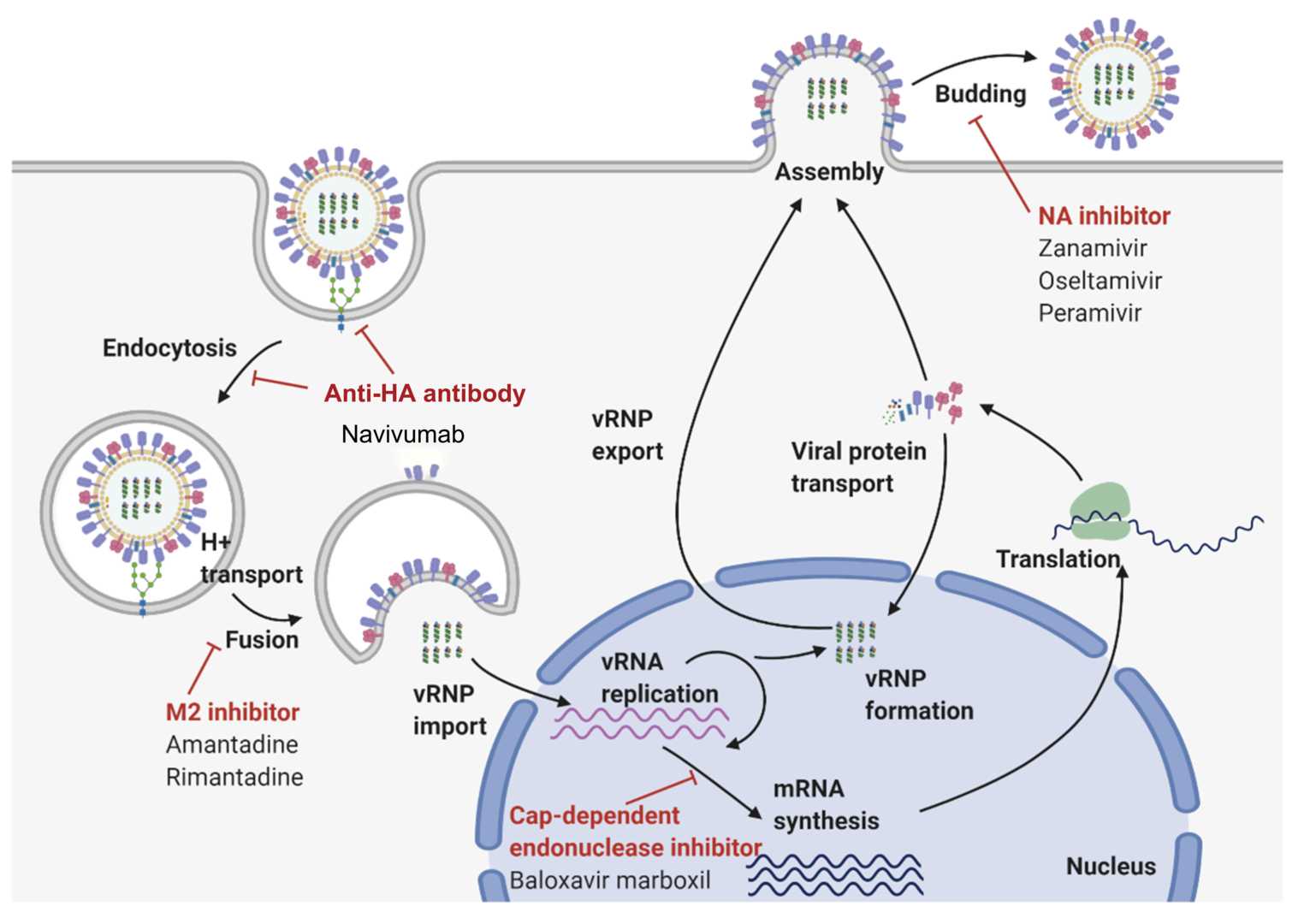 Figure 1. The Mechanism of Action of Navivumab1,2.
Figure 1. The Mechanism of Action of Navivumab1,2.
Related Clinical Applications of Navivumab
Navivumab has shown promising therapeutic potential in both preclinical studies and clinical trials, particularly in animal models of influenza infection. In mice, Navivumab significantly increased survival rates after infection with Influenza A Virus (IAV), suggesting that early treatment with this monoclonal antibody could be effective, especially for patients at high risk of severe illness, such as the elderly or those with weakened immune systems.
Beyond treatment, Navivumab also holds promise as a preventive measure against influenza. By providing passive immunity, Navivumab can offer immediate protection when administered before exposure to the virus. This is especially valuable for high-risk populations, including the elderly, immunocompromised individuals, or healthcare workers during flu season. Giving the antibody during peak flu periods could potentially reduce both the frequency and severity of infections, acting as an additional line of defense.
Additionally, combining Navivumab with antiviral medications like Oseltamivir has shown synergistic effects. Oseltamivir, which blocks the release of new viral particles, can work alongside Navivumab that prevent the virus from entering host cells. Together, these therapies form a comprehensive blockade of the virus's lifecycle, making the treatment more effective—especially against strains that might be resistant to single therapies. This combination approach could offer an enhanced strategy for tackling influenza infections.
Clinical Projects of CT-P27 (the Combination of CT-P22 and CT-P23) *
| NCT ID | Study Title | Study Status | Conditions | Sponsor | Start Date |
| NCT02071914 | A Study to Evaluate the Efficacy and Safety of CT-P27 in an Influenza Challenge Model | COMPLETED | Influenza A | Celltrion | 2014/2/15 |
| NCT03511066 | A Study to Evaluate the Efficacy and Safety of CT-P27 in Acute Uncomplicated Influenza A Infection | TERMINATED | Influenza A | Celltrion | 2016/12/9 |
* The table was excerpted from the following website: https://clinicaltrials.gov/search?cond=CT-P27
- Jung, Hi Eun, and Heung Kyu Lee. "Host protective immune responses against influenza A virus infection." Viruses 12.5 (2020): 504.
- The image retrieved from Figure 2 "Host protective immune responses against influenza A virus infection." Jung, Hi Eun, 2020, is used under [CC BY 4.0]. The image was modified by adding the text "Anti-HA antibody" and "Navivumab" and the title was changed to "The Mechanism of Action of Navivumab."
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
