Rivabazumab Overview
Introduction of Rivabazumab
Rivabazumab is a recombinant antibody specifically designed to target the pcrV protein of Pseudomonas aeruginosa (P. aeruginosa ). This antibody represents a promising approach in combating P. aeruginosa infections, particularly in increasing antibiotic resistance. Initially developed by KaloBios Pharmaceuticals, rivabazumab pegol (KB001) is a pegylated version of the antibody. In 2013, the U.S. Food and Drug Administration granted orphan drug designation to KB001 for treating cystic fibrosis (CF) patients with Pseudomonas aeruginosa infections.
Biological and Chemical Properties of Type III Secretion Protein PcrV
Protein Structure
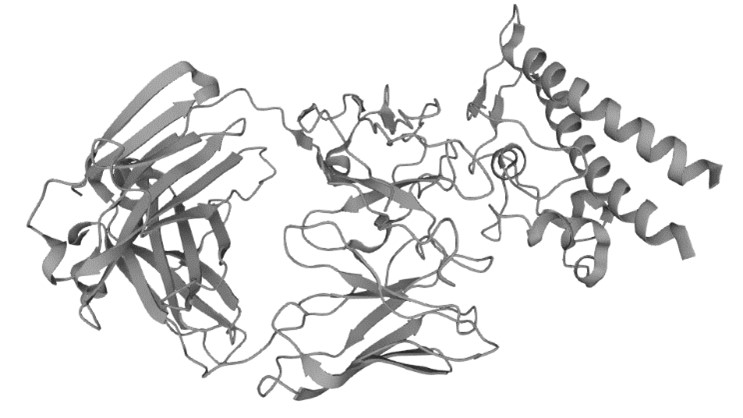 Figure 1. The Structure of Type III Secretion Protein PcrV (UniProt)1,2
Figure 1. The Structure of Type III Secretion Protein PcrV (UniProt)1,2
The Mechanism of Rivabazumab Action
Rivabazumab exerts its therapeutic effects by specifically binding to the pcrV protein of P. aeruginosa, thereby inhibiting its function within the Type III secretion system (T3SS). The T3SS is a multi-protein complex employed by numerous pathogenic Gram-negative bacteria, including Salmonella, Shigella, Yersinia, Pseudomonas, Vibrio, and Escherichia. This system is pivotal for bacterial virulence, facilitating the evasion of host immune responses and promoting bacterial survival and proliferation. The pcrV protein is a crucial component of P. aeruginosa's T3SS. Structurally, it forms a needle-like appendage that regulates the translocation of effector proteins into the host cytoplasm. In normal conditions, pcrV functions as part of the T3SS to manipulate host cellular processes, facilitating bacterial colonization and infection. However, in pathological scenarios, the overexpression or hyperactivation of the T3SS can enhance P. aeruginosa virulence, leading to severe tissue damage, inflammation, and compromised host defenses. This contributes to chronic infections, particularly in immunocompromised individuals and those with underlying conditions like cystic fibrosis or chronic wounds.
Upon administration, rivabazumab targets the infected tissue and binds specifically to the pcrV protein with its antigen-binding site, forming a stable antigen-antibody complex. This complex effectively blocks the T3SS apparatus and prevents the translocation of effector proteins into host cells, thus neutralizing bacterial virulence mechanisms. As a result, P. aeruginosa's virulence is reduced, leading to enhanced bacterial clearance by the host immune system. Additionally, the compromised infection machinery limits bacterial growth and dissemination, contributing to a favorable therapeutic outcome.
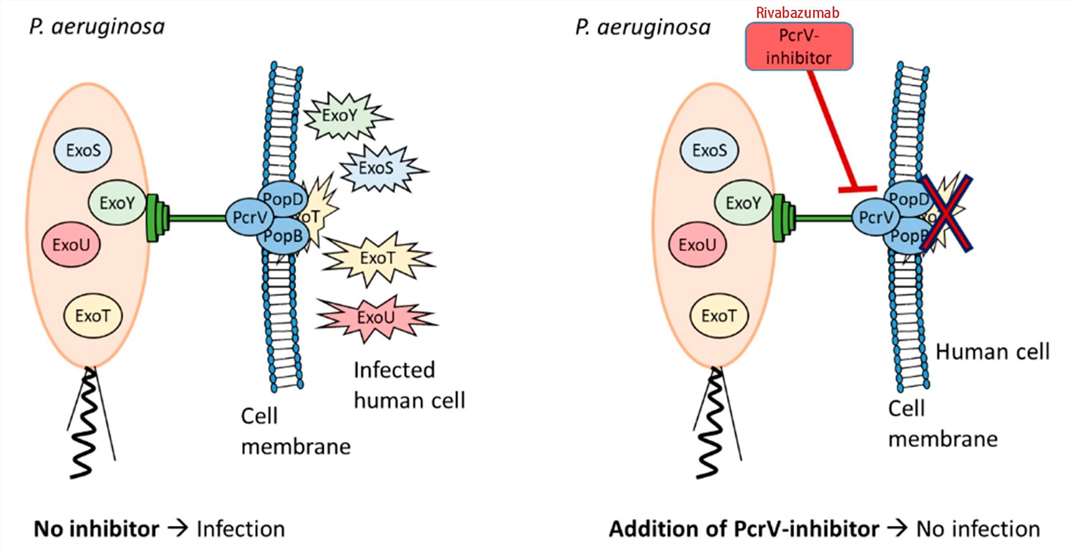 Figure 2. The Mechanism of Rivabazumab Action3,4
Figure 2. The Mechanism of Rivabazumab Action3,4
Clinical Projects of Rivabazumab pegol (KB001) *
| NCT ID | Study Title | Study Status | Conditions | Sponsor | Start Date |
| NCT01695343 | Study to Evaluate the Effect of KB001-A on Time-to-Need for Antibiotic Treatment | COMPLETED | Cystic Fibrosis | Humanigen, Inc. | 2012-12 |
| NCT00691587 | Pilot Trial of KB001 in Mechanically-Ventilated Patients Colonized with Pseudomonas Aeruginosa | COMPLETED |
Pseudomonas Aeruginosa Ventilator Associated Pneumonia |
Humanigen, Inc. | 2008-04 |
| NCT00638365 | Dose Escalation Study of KB001 in Cystic Fibrosis Patients Infected With Pseudomonas Aeruginosa | COMPLETED | Cystic Fibrosis | Humanigen, Inc. | 2008-03 |
* The table is excerpted from the following website: https://clinicaltrials.gov/search?cond=KB001-A
What We Provide
Anti-Pseudomonas Aeruginosa pcrV Recombinant Antibody Fab Fragment (Rivabazumab)
Creative Biolabs offers high-quality rivabazumab, specifically designed for use in Enzyme-Linked Immunosorbent Assay, Immunohistochemistry, Immunofluorescence, Immunoprecipitation, Flow Cytometry and Functional Studies. This product is designed for lab research use only, and is not suitable for diagnostic, therapeutic, or any in vivo human use.
- Immunogen
- The details of the immunogen for this antibody are not available.
- Host Species
- Human
- Derivation
- Humanized
- Type
- Fab' G1-κ
- Specificity
- Pseudomonas aeruginosa type III secretion system (TTSS)PcrV protein
- Species Reactivity
- Pseudomonas aeruginosa
- Applications
- Used for immunoassay techniques such as: Enzyme-Linked Immunosorbent Assay; Immunohistochemistry; Immunofluorescence; Immunoprecipitation; Flow Cytometry; Functional Studies
- Conjugate
- Unconjugated
- CAS
- 1613506-32-3
- Generic Name
- Rivabazumab
- UNII
- T3V24FDA87
- Related Disease
- Pseudomonas aeruginosa infections
- Uniprot Database (https://www.uniprot.org/uniprotkb/G3XD49/entry#function)
- The image was retrieved from UniProt Database and used under [CC BY 4.0]. It was not modified and the title was "The Structure of Type III Secretion Protein PcrV".
- Sundin, Charlotta et al. "Identification of Small Molecules Blocking the Pseudomonas aeruginosa type III Secretion System Protein PcrV." Biomolecules vol. 11,1 55. 4 Jan. 2021.
- Images retrieved from Figure 1 " Identification of Small Molecules Blocking the Pseudomonas aeruginosa type III Secretion System Protein PcrV." Sundin, Charlotta, 2021, used under [CC BY 4.0] (https://creativecommons.org/licenses/by/4.0/). The image was modified by adding the text "Rivabazumab" and the title was changed to " The Mechanism of Rivabazumab Action".
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



