Teneliximab Overview
Introduction of Teneliximab
Teneliximab, also known as Chi220 or BMS-224819, is a chimeric antibody of IgGl isotype. This antibody blocks the CD40-CD40L interaction, but is characterized as a partial agonist as it can co-stimulate with anti-IgM to induce low levels of normal B-cell proliferation. Teneliximab depletes peripheral blood B-cells in NHPs, inhibits primary and secondary antibody responses against T-cell-dependent antigens. Teneliximab alone prolonged renal allograft survival in cynomolgus monkeys. In islet allotransplantation, teneliximab alone only gave short term, transient graft survival benefit; a combination of teneliximab and CTLA4-Ig, however, resulted in long-term graft survival. Mechanistically, CD40-CD40L co-stimulation blockade rather than B-cell depletion might be the key mechanism for teneliximab, as the potent B-cell depleting agent rituximab did not provide the allograft survival benefit seen with teneliximab. At present, clinical development plan for teneliximab has not yet been disclosed.
Mechanism of Action of Teneliximab
Unlike conventional immunosuppressive therapies such as steroids and calcineurin inhibitors, co-stimulation blockade-based therapies minimize systemic toxicity and generalized attenuation of protective immunity while specifically targeting the recipient response to donor antigen. Engraftment and long-term survival of porcine islets in a diabetic nonhuman primate (NHP) model was successfully achieved in two landmark proof-of-concept studies using a co-stimulation blockade-based strategy targeting CD154; however, the association of anti-CD154 therapy with thromboembolic phenomena, an effect thought to occur by CD40-independent mechanisms, has stifled its clinical development. While enthusiasm for CD154-specific antibodies has predictably waned, the CD40/CD154 interaction remains a promising target. CD40-directed therapy might avoid CD154-associated thrombotic sequelae while bestowing comparable xenograft protection.
CD40 belongs to the tumor necrosis factor (TNF) superfamily and are expressed on a wide range of tissues and cell types, with CD40 constitutively expressed on antigen-presenting cells (APC), including B cells, macrophages and dendritic cells (DC), and CD154 inducibly expressed mainly on CD4+ T cells and endothelial cells following activation. CD40 binds its ligand CD40L, which is transiently expressed on T cells and other non-immune cells under inflammatory conditions. A wide spectrum of molecular and cellular processes is regulated by CD40 engagement including the initiation and progression of cellular and humoral adaptive immunity. Anti-CD40 therapy represents an alternate strategy targeting the important CD40/CD154 T cell activation pathway. Teneliximab is thought to achieve its immunosuppressive effects through a combination of (a) activation-induced cell energy or apoptosis resulting from a weak partial agonist effect of CD40 ligation and (b) antibody-dependent cellular cytotoxicity resulting in depletion of CD40-expressing B-cells and other APCs. Demonstrating that the therapeutic effects of teneliximab are not due to B cell depletion alone, some studies showed that animals treated with rituximab (an anti-CD20 antibody) instead of teneliximab rapidly rejected their islet allografts despite profound B cell depletion. In addition, graft survival following a short course of teneliximab often persisted long after B cell repopulation, implicating its downstream, non-deletional immunoregulatory effects. It is likely that cellular depletion plays a crucial though incompletely understood role in promotion of transplantation survival.
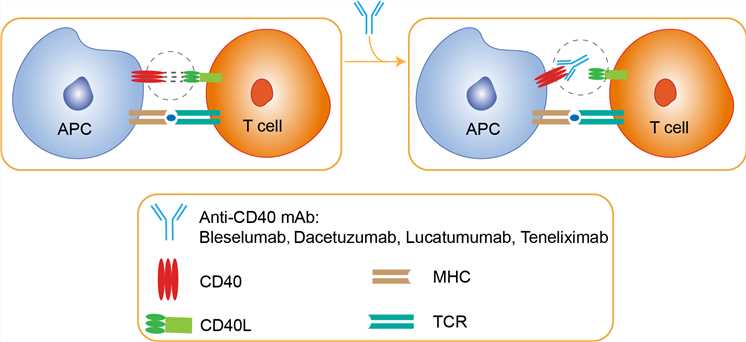
Fig 1. Mechanism of Action of Teneliximab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



